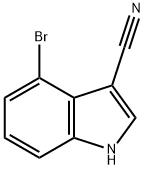
4-BROMO-3-CYANOINDOLE synthesis
- Product Name:4-BROMO-3-CYANOINDOLE
- CAS Number:903131-13-5
- Molecular formula:C9H5BrN2
- Molecular Weight:221.05
52488-36-5
474 suppliers
$15.00/1g
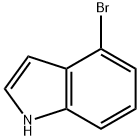
1189-71-5
347 suppliers
$18.00/5g

903131-13-5
45 suppliers
inquiry
Yield:903131-13-5 87%
Reaction Conditions:
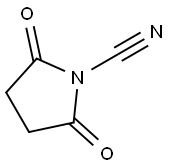
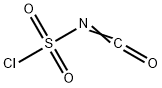
Stage #1: 4-bromo-1H-indole;isocyanate de chlorosulfonyle in acetonitrile at 0; for 2.08333 h;
Stage #2: with triethylamine in acetonitrile at 0 - 20;
Steps:
14 Synthesis of 4-bromo-1H-indole-3-carbonitrile (LVII)
Example 14 Synthesis of 4-bromo-1H-indole-3-carbonitrile (LVII) To a 0° C. solution of 4-bromoindole (5 g, 25.5 mmol) in acetonitrile (50 ml) was added dropwise chlorsulfonylisocyanate (2.37 ml, 27.3 mmol) over a 5 minute period. The reaction mixture was stirred at 0° C. for 2 hours, and TEA (3.77 ml, 27.0) was added dropwise over a 10 minute period. The reaction mixture was allowed reach room temperature overnight, poured into ice/water mixture (200 ml), and the aqueous layer was extracted with EtOAc. The combined organic extracts were dried over Na2SO4, filtered, concentrated, and purified by flash chromatography to afford 4-bromo-1H-indole-3-carbonitrile LVII as an off-white solid (4.9 g, 87% yield).
References:
US2007/123527,2007,A1 Location in patent:Page/Page column 68