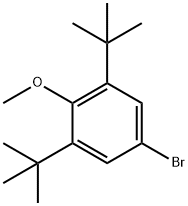
4-Bromo-2,6-di-tert-butylanisole synthesis
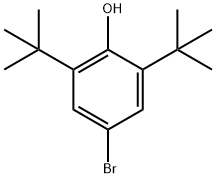
- Product Name:4-Bromo-2,6-di-tert-butylanisole
- CAS Number:1516-96-7
- Molecular formula:C15H23BrO
- Molecular Weight:299.25
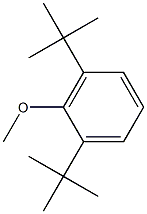
1516-95-6
13 suppliers
$41.89/10g
3848-49-5
1 suppliers
inquiry
Yield:3848-49-5 96%
Reaction Conditions:
with NBS in acetonitrile; for 20 h;
Steps:
7 5-bromo-l,3-di-tert-butyl-2-methoxybenzene
l,3-di-tert-butyl-2-methoxybenzene (Bai, Xinyan et al, Tetrahedron, 69(3), 1105-1111; 2013 (2.0 g, 9.1 mmol) was dissolved in acetonitrile (30.0 mL) and treated with n-bromosuccinimide (1.7 g, 9.5 mmol) and the mixture allowed to stir for 20 hours. The mixture was concentrated in vacuo then filtered through a Si02 plug with hexanes. The solvent was then removed in vacuo to provide an oil (2.6g, 96%) used without further purification. 1H NMR (500 MHz, Chloroform-d) d 7.34 (s, 2H), 3.68 (s, 3H), 1.41 (s, 18H).
References:
WO2020/150668,2020,A1 Location in patent:Paragraph 0191