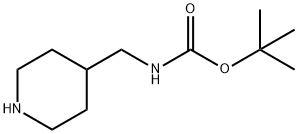
4-(Boc-Aminomethyl)piperidine synthesis
- Product Name:4-(Boc-Aminomethyl)piperidine
- CAS Number:135632-53-0
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
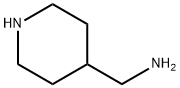
7144-05-0
217 suppliers
$6.00/1g
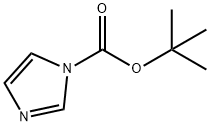
49761-82-2
156 suppliers
$5.00/1g

135632-53-0
238 suppliers
$21.00/1g
Yield:135632-53-0 70%
Reaction Conditions:
in toluene at 25;
Steps:
227
A solution of piperidinyl-4-methylamine (3.6 g) and N-tert-butoxycarbonylimidazole (5.3 g) in toluene (80 mL) was stirred at 25° C. overnight. The solution was then concentrated and the resultant residue was purified by column chromatography on silica gel (EtOAc/Hexane=1/2) to give Intermediate 227-I (4.7 g) in a 70% yield. Intermediate 227-I (4.7 g) and Et3N (2.7 mL) in 1-pentanol (20 mL) was reacted with 2,4-dichloro-6-aminopyrimidine (5.4 g) at 120° C. for 12 hours. After the solvent was removed, the residue was purified by column chromatography on silica gel (EtOAc/Hexane=1/9) to afford Intermediate 227-II (5.2 g) in a 70% yield. A solution of Intermediate 227-II (1.0 g) treated with 1 M HCl (20 mL) in CH2Cl2 (10 mL) was stirred at room temperature for 8 hours. After the solution was concentrated, the resultant residue was neutralization with NH4OH, and extracted with CH2Cl2. The organic layer was separated and concentrated. The residue thus obtained was purified by column chromatography on silica gel (using MeOH as an eluant) to afford Intermediate 227-III (636 mg) in a 90% yield. Intermediate 222-III (790 mg) prepared from Example 222 was added to a solution of Intermediate 227-III (450 mg) in MeOH (20 mL). The mixture was stirred at 25° C. for 2 hours. NaBH(OAc)3 (2.0 g) was then added at 25° C. for 12 hours. After the solution was concentrated, a saturated aq. NaHCO3 solution was added to the resultant residue. The mixture was then extracted with CH2Cl2. The organic layer was separated and concentrated. The residue thus obtained was purified by column chromatography on silica gel (using MeOH as an eluant) to afford Intermediate 227-IV (539 mg) in a 60% yield. N1-Morpholine-N1-piperazine ethane (240 mg) was added to a solution of Intermediate 227-IV (160 mg) in 1-pentanol (1 mL). The mixture was stirred at 120° C. for 8 hours. The solution was concentrated and the residue was purified by column chromatography on silica gel (EtOAc/MeOH=5/1) to afford Intermediate 227-V (85 mg) in a 40% yield. A solution of 20% TFA/CH2Cl2 (1 mL) was added to a solution of Intermediate 227-V (85 mg) in CH2Cl2 (1 mL). The reaction mixture was stirred for 8 hours at room temperature and concentrated by removing the solvent. The resultant residue was purified by column chromatography on silica gel (21% NH3 (aq)/MeOH=1/19) to afford Compound 227 (65 mg) in a 90% yield. Compound 227 was then treated with 1 M HCl (1 mL) in CH2Cl2 (1 mL) for 0.5 hour. After the solvents were removed, the residue was treated with ether and filtered to give hydrochloride salt of Compound 227. CI-MS (M++1): 544.4.
References:
Yen, Chi-Feng;Hu, Cheng-Kung;Chou, Ming-Chen;Tseng, Chen-Tso;Wu, Chien-Huang;Huang, Ying-Huey;Chen, Shu-Jen;King, Chi-Hsin Richard US2006/281712, 2006, A1 Location in patent:Page/Page column 108-109
![TERT-BUTYL N-[(1-BENZYL-4-PIPERIDINYL)METHYL]CARBAMATE](/CAS/GIF/173340-23-3.gif)
173340-23-3
25 suppliers
$270.00/1g

135632-53-0
238 suppliers
$21.00/1g

24424-99-5
863 suppliers
$13.50/25G

7144-05-0
217 suppliers
$6.00/1g

135632-53-0
238 suppliers
$21.00/1g
39546-32-2
364 suppliers
$6.00/5g

135632-53-0
238 suppliers
$21.00/1g
375355-32-1
3 suppliers
inquiry

135632-53-0
238 suppliers
$21.00/1g
