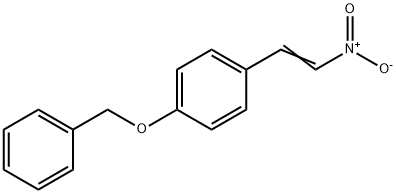
4-BENZYLOXY-TRANS-BETA-NITROSTYRENE 97 synthesis
- Product Name:4-BENZYLOXY-TRANS-BETA-NITROSTYRENE 97
- CAS Number:2982-55-0
- Molecular formula:C15H13NO3
- Molecular Weight:255.27
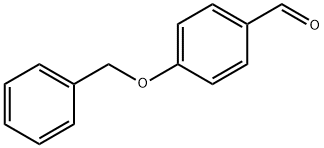
4397-53-9
335 suppliers
$8.00/10g

75-52-5
0 suppliers
$35.28/100g

2982-55-0
27 suppliers
$50.00/250mg
Yield:2982-55-0 100%
Reaction Conditions:
Stage #1: p-benzyloxybenzaldehyde;nitromethanewith sodium methylate in methanol at 0 - 20; for 0.166667 h;
Stage #2: with hydrogenchloride;water in methanol at 0 - 20; for 0.25 h;
Steps:
1.1.1
Manufacturing Example 1-1-1 1-Benzyloxy-4-((E)-2-nitro-vinyl)-benzene; To a mixture of 4-benzyloxybenzaldehyde (1.0 g, 4.7 mmol) and sodium methoxide (28% methanol solution, 150 μL, 0.74 mmol) and methanol (10 mL) were added nitromethane (330 μL, 6.1 mmol) and sodium methoxide (28% methanol solution, 1.0 mL, 4.9 mmol) at 0° C., which was stirred for 10 minutes at room temperature. The reaction mixture was cooled to 0° C., and 5 N aqueous hydrochloric acid solution (20 mL) was added thereto at the same temperature. The reaction mixture was then stirred for 15 minutes at room temperature. The precipitated solids were filtered to obtain the title compound (1.2 g, 100%).1H-NMR Spectrum (DMSO-d6) δ (ppm): 5.20 (2H, s), 7.10-7.14 (2H, m), 7.32-7.48 (5H, m), 7.82-7.85 (2H, m), 8.12 (2H, dd, J=13.5, 18.2 Hz).
References:
US2009/82403,2009,A1 Location in patent:Page/Page column 50

75-52-5
0 suppliers
$35.28/100g
1700-37-4
249 suppliers
$9.00/1g

2982-55-0
27 suppliers
$50.00/250mg
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
75 suppliers
$28.60/100MG

2982-55-0
27 suppliers
$50.00/250mg

4397-53-9
335 suppliers
$8.00/10g

2982-55-0
27 suppliers
$50.00/250mg
![ETHYL 3-[4-(BENZYLOXY)PHENYL]ACRYLATE](/StructureFile/ChemBookStructure4/GIF/CB4167514.gif)
104315-07-3
26 suppliers
$155.00/100mg

2982-55-0
27 suppliers
$50.00/250mg
