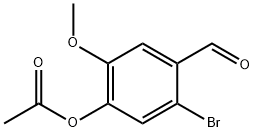
4-ACETOXY-2-BROMO-5-METHOXYBENZALDEHYDE 98 synthesis
- Product Name:4-ACETOXY-2-BROMO-5-METHOXYBENZALDEHYDE 98
- CAS Number:52783-83-2
- Molecular formula:C10H9BrO4
- Molecular Weight:273.08
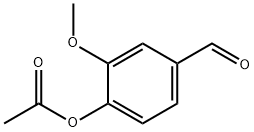
881-68-5
283 suppliers
$5.00/5g

52783-83-2
37 suppliers
$21.00/1g
Yield:52783-83-2 95%
Reaction Conditions:
with bromine;potassium bromide in lithium hydroxide monohydrate;acetonitrile at 20; for 20 h;
Steps:
4-Acetoxy-6-bromo-3-methoxybenzaldehyde (18)
Br2 (9.52 g, 59.9 mmol) was cautiously dropped into a solution of 17 (3.90 g, 19.9 mmol) and KBr (8.03 g, 67.5 mmol) in MeCN/H2O (1:1, v/v, 150 mL). The system was firmly closed and stirred for 20 h at rt, when complete consumption of the starting material was assessed. Then, the mixture was poured onto crushed ice (100 g), the solids were filtered, washed with H2O, and further dissolved in EtOAc (300 mL). The organic solution was washed with H2O (250 mL) and brine (2 × 100 mL), and dried (MgSO4). The volatiles were removed in vacuo to afford 18 as an orange solid; yield: 5.13 g (95%). 1H NMR (300 MHz, CDCl3): δ = 10.27 (s, 1 H, CHO), 7.51 (s, 1 H, H-2),7.36 (s, 1 H, H-5), 3.89 (s, 3 H, 3-OCH3), 2.33 (s, 3 H, 2'- CH3). 13C NMR (75 MHz, CDCl3): δ = 191.0 (CHO), 168.1 (1'-CO2), 151.4 (C-3), 145.1 (C-4), 131.7 (C-1), 128.1 (C-5), 118.0 (C-6), 112.3 (C-2), 56.4 (3-OCH3), 20.7 (2'-CH3). These data were in accordance with the literature values.33
References:
Aguilar, Abel A. Arroyo;Kaufman, Teodoro S.;Larghi, Enrique L.;Ledesma, Gabriela N.;Tirloni, Bárbara [Synthesis,2019,vol. 51,# 22,p. 4253 - 4262]
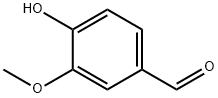
121-33-5
1146 suppliers
$9.00/1g

52783-83-2
37 suppliers
$21.00/1g