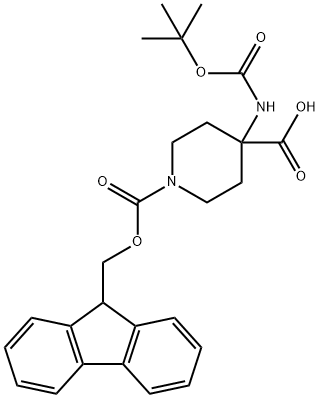
4-(Boc-amino)-1-(Fmoc-piperidinyl)-4-carboxylic Acid synthesis
- Product Name:4-(Boc-amino)-1-(Fmoc-piperidinyl)-4-carboxylic Acid
- CAS Number:368866-07-3
- Molecular formula:C26H30N2O6
- Molecular Weight:466.53
252720-31-3
109 suppliers
$45.00/100mg
28920-43-6
535 suppliers
$5.00/5g

368866-07-3
148 suppliers
$60.00/250mg
Yield:368866-07-3 89%
Reaction Conditions:
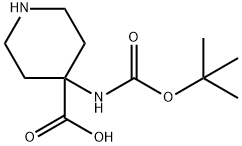
Stage #1: 4-[(2-methylpropan-2-yl)oxycarbonylamino]piperidine-4-carboxylic acidwith anhydrous sodium carbonate in 1,4-dioxane;lithium hydroxide monohydrate at 25; for 0.5 h;Inert atmosphere;
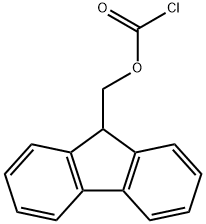
Stage #2: 9H-fluoren-9-ylmethyl carbonochloridate in 1,4-dioxane;lithium hydroxide monohydrate at 25;Inert atmosphere;
Steps:
1.b A 4-|(toT-Butoxycarbony l)amino|-l-|(9H-fluoren-9- ylmethoxy)carbonyl]piperidine-4-carboxylic acid.
To a solution of 4-[(tert- butoxycarbonyl)amino]piperidine-4-carboxylic acid (500.0 mg, 2.047 mmol) in dioxane (8.0 mL) was added a solution of Na2C03 (282.1 mg, 2.661 mmol) in H20 (8.0 mL) and was stirred for 30 min at 25 °C. Then fluorenylmethyloxycarbonyl chloride (582.4 mg, 2.251 mmol) was added and was stirred for 1 overnight at 25 °C. The resulting solution was diluted with 20 mL of 0. The resulting solution was extracted with EtOAc. The pH value of the solution was adjusted to 3 with HC1 (1 mmol/L). The solids were collected by filtration. This resulted in 858 mg (89%) of 4-[(tert-butoxycarbonyl)amino]-l-[(9H-fluoren-9- ylmethoxy)carbonyl]piperidine-4-carboxylic acid as a white solid. LCMS (ESI): [M+H]+ =467.
References:
WO2022/150314,2022,A1 Location in patent:Paragraph 00276; 00283

252720-31-3
109 suppliers
$45.00/100mg
82911-69-1
624 suppliers
$7.00/25g

368866-07-3
148 suppliers
$60.00/250mg

24424-99-5
839 suppliers
$13.50/25G

368866-07-3
148 suppliers
$60.00/250mg
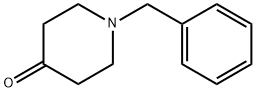
3612-20-2
488 suppliers
$6.00/10g

368866-07-3
148 suppliers
$60.00/250mg
![8-BENZYL-1,3,8-TRIAZASPIRO[4.5]DECANE-2,4-DIONE](/StructureFile/ChemBookStructure3/GIF/CB9137202.gif)
28936-94-9
70 suppliers
$45.00/50mg

368866-07-3
148 suppliers
$60.00/250mg