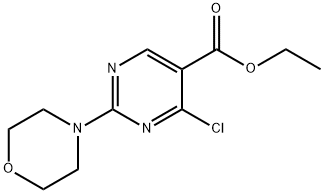
ETHYL 4-CHLORO-2-MORPHOLINOPYRIMIDINE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 4-CHLORO-2-MORPHOLINOPYRIMIDINE-5-CARBOXYLATE
- CAS Number:34750-23-7
- Molecular formula:C11H14ClN3O3
- Molecular Weight:271.7
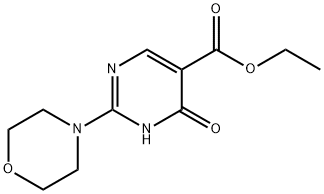
25693-41-8
19 suppliers
$20.00/250mg

34750-23-7
18 suppliers
$32.00/250mg
Yield:34750-23-7 96.5%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 0.333333 h;
Steps:
15
Intermediate 15. 4-Chloro-2-morpholin-4-yl-pyrimidine-5-carboxylic acid ethyl ester. To a room temperature solution of Intermediate 4 (3.46 g, 13.7 mmol) and DMF (-5 drops) in anhydrous dichloromethane (70 mL) was added oxalyl chloride (1.31 mL, 15.0 mmol) dropwise via syringe. The reaction effervesced. The reaction was stirred at room temperature for twenty minutes. HPLC analysis of an aliquot indicated complete consumption of the starting pyrimidine and the formation of a new, less-polar product. The reaction was diluted with saturated aqueous NaHC03. The dilution was extracted three times with dichloromethane. The extracts were combined, washed with brine, dried over Na2S04, filtered through celite, and concentrated to give the desired title product (1.056 g, 96.5% yield), as a light yellow solid.
References:
WO2005/42519,2005,A1 Location in patent:Page/Page column 41

25693-41-8
19 suppliers
$20.00/250mg

34750-23-7
18 suppliers
$32.00/250mg
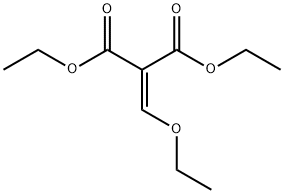
87-13-8
445 suppliers
$5.00/5g

34750-23-7
18 suppliers
$32.00/250mg
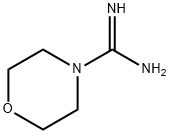
17238-66-3
54 suppliers
inquiry

34750-23-7
18 suppliers
$32.00/250mg
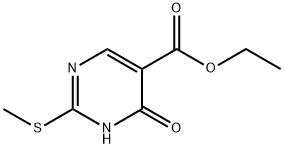
53554-29-3
200 suppliers
$6.00/1g

34750-23-7
18 suppliers
$32.00/250mg