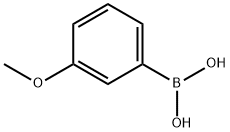
3-Methoxyphenylboronic acid synthesis
- Product Name:3-Methoxyphenylboronic acid
- CAS Number:10365-98-7
- Molecular formula:C7H9BO3
- Molecular Weight:151.96
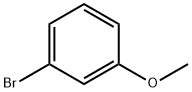
2398-37-0
584 suppliers
$8.00/10g
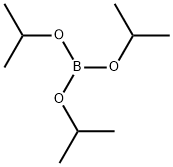
5419-55-6
388 suppliers
$12.00/5g

10365-98-7
425 suppliers
$9.00/10g
Yield:10365-98-7 156.1 g
Reaction Conditions:
Stage #1: 3-methoxyphenyl bromidewith n-butyllithium in tetrahydrofuran;hexane at -70; for 1 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at 20; for 18 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water;
Steps:
1 First Step: Preparation of 3-methoxyphenylboronic acid (T-2)
n-Butyllithium in n-hexane (925.0 mL, 1.59 M) was added dropwise to a mixture of THF (1,000 mL) and 1-bromo-3-methoxybenzene (T-1) (250.0 g, 1.34 mol) at -70° C. under an atmosphere of nitrogen.
After the addition had been completed, the reaction mixture was stirred at the same temperature for 1 hour. Triisopropyl borate (300.9 g, 1.60 mol) was added dropwise, and then the mixture was warmed slowly to room temperature.
The stirring was continued at room temperature for 18 hours, and the reaction mixture was poured into 6M-hydrochloric acid. The mixture was extracted with ethyl acetate (500 mL) four times, and the organic layer was washed with water. After the organic layer had been dried over anhydrous magnesium sulfate, the organic solvent was distilled off under reduced pressure.
The residue was sufficiently washed with heptane to leave colorless solids (156.1 g) of 3-methoxyphenylboronic acid (T-2).
References:
US8394468,2013,B2 Location in patent:Page/Page column 52; 53

2398-37-0
584 suppliers
$8.00/10g

10365-98-7
425 suppliers
$9.00/10g

2398-37-0
584 suppliers
$8.00/10g
121-43-7
376 suppliers
$14.00/25mL

10365-98-7
425 suppliers
$9.00/10g
325142-84-5
115 suppliers
$6.00/1g

10365-98-7
425 suppliers
$9.00/10g

13675-18-8
165 suppliers
$6.00/1g

2398-37-0
584 suppliers
$8.00/10g

10365-98-7
425 suppliers
$9.00/10g