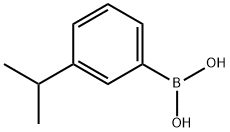
3-ISOPROPYLPHENYLBORONIC ACID synthesis
- Product Name:3-ISOPROPYLPHENYLBORONIC ACID
- CAS Number:216019-28-2
- Molecular formula:C9H13BO2
- Molecular Weight:164.01
5419-55-6
388 suppliers
$12.00/5g
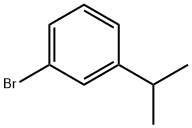
5433-01-2
123 suppliers
$27.00/5G

216019-28-2
183 suppliers
$7.00/250mg
Yield:-
Reaction Conditions:
Stage #1:1-bromo-3-isopropylbenzene with n-butyllithium in tetrahydrofuran at -70; for 0.75 h;Inert atmosphere;
Stage #2:Triisopropyl borate in tetrahydrofuran at -70 - 0; for 2 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at -20;
Steps:
Preparation of sulfonyl chlorides:Preparation of 5-(3-Isopropyl-phenyl)-pyridine-2-sulfonyl chloride; 3-Isopropyl-phenylboronic acid; n-BuLi (7.7 mL, 14.0 mmol) was added dropwise to a stirred solution of 1-bromo-3-isopropyl-benzene (2.4 g, 12.1 mmol) in dry THF (40 mL) over 30 min at -70° C. under nitrogen. The reaction mixture was degassed for 15 min, and triisopropylborate (2.63 g, 14.0 mmol) was added at the same temperature. The reaction mixture was gradually warmed to 0° C. during 90 min and stirred for an additional 30 min at 0° C. The reaction mixture was then cooled to -20° C., and an aqueous solution of 2N HCl (20 mL) was added slowly. THF was distilled off and the reaction mixture was extracted with ethyl acetate (2×20 mL). The combined organic layers were dried over Na2SO4, and concentrated in vacuo to obtain the crude product (1.85 g, 93.5%), which was used for the next step without further purification.
References:
Firooznia, Fariborz;Gillespie, Paul;Goodnow, JR., Robert Alan;Lin, Tai-An;Sidduri, Achyutharao;So, Sung-Sau;Tan, Jenny US2010/41713, 2010, A1 Location in patent:Page/Page column 19-20

5433-01-2
123 suppliers
$27.00/5G

216019-28-2
183 suppliers
$7.00/250mg