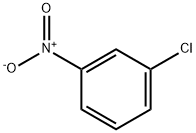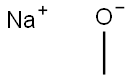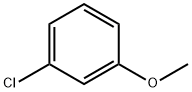
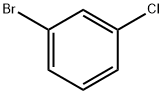
3-Chloroanisole synthesis
- Product Name:3-Chloroanisole
- CAS Number:2845-89-8
- Molecular formula:C7H7ClO
- Molecular Weight:142.58
108-37-2
353 suppliers
$6.00/10g
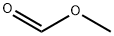
107-31-3
347 suppliers
$16.00/25mL

2845-89-8
291 suppliers
$6.00/5g
Yield:2845-89-8 99%
Reaction Conditions:
with copper(I) bromide in methanol;sodium methylate
Steps:
3 Methoxylation of 3-Chlorobromobenzene to yield 3-Chloroanisole
EXAMPLE 3 Methoxylation of 3-Chlorobromobenzene to yield 3-Chloroanisole 3-Chlorobromobenzene (19.2 g, 0.1 mole) was dissolved in a solution of sodium methoxide (0.4 mole), in methanol (100 ml), and this reaction mixture was flushed with nitrogen. Methyl formate (3.6 g, 0.06 mole) was added, followed by cuprous bromide (2.9 g, 0.02 mole). The resultant suspension was refluxed for 9 hours, until the reaction was judged by TLC to be complete. The crude reaction mixture was acidified, and extracted into isopropyl acetate. The solvent was removed by evaporation at 20° C., to give a pale yellow liquid (14.1 g; 99%), whose infra-red spectrum was identical to that of authentic 3-chloroanisole as recorded in the Aldrich catalogue. GLC analysis showed one product peak, with a higher retention time than the starting material (Bentone/OVI, 180°). GC-MS showed no detectable 3-bromoanisole and only a very small quantity of dimethylresorcinol.
References:
Sterling Drug Inc. US4495353, 1985, A
10365-98-7
418 suppliers
$9.00/10g

2845-89-8
291 suppliers
$6.00/5g
150-19-6
343 suppliers
$6.00/5g

2845-89-8
291 suppliers
$6.00/5g

124-38-9
130 suppliers
$175.00/23402
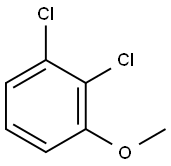
1984-59-4
212 suppliers
$20.00/25g

2845-89-8
291 suppliers
$6.00/5g
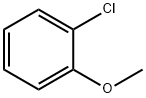
766-51-8
214 suppliers
$20.00/25g
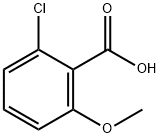
3260-89-7
84 suppliers
$50.00/1g
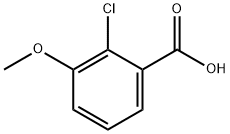
33234-36-5
107 suppliers
$12.00/100mg