
3-Chloro-1-propanol synthesis
- Product Name:3-Chloro-1-propanol
- CAS Number:627-30-5
- Molecular formula:C3H7ClO
- Molecular Weight:94.54
Reaction: 250 g of trimethylene glycol are shaken with 450 g of sulfur chloride, the mixture becoming warm, sulfur dioxide being rapidly evolved, and sulfur deposited. This reaction continues for 1 hours without heating, whereafter the mixture is heated for a further 6 hours on the water-bath and then for 30 min, over a free flame. When no more sulfur dioxide is evolved, the mixture is allowed to cool, then extracted several times with ether. The sulfur is washed with ether, and the united ethereal layers are shaken with sodium carbonate solution to remove sulfur dioxide, dried over sodium sulfate, and freed from ether. The residue is fractionated and the fraction of b.p. 140-180° C is redistilled, giving 60% (160 g) of 3-chloro-1-propanol, b.p. 160-164°C/760 mm, 60-64° C/10 mm.
J. Amer. Chem. Soc, 38, 2481 (1916)
J. Chem. Soc, 1928, 2439

504-63-2
543 suppliers
$9.00/5g

627-30-5
319 suppliers
$9.00/1g
Yield:627-30-5 96%
Reaction Conditions:
with hydrogenchloride;benzenesulfonic acid in lithium hydroxide monohydrate at 50 - 90; for 13 h;Large scale;
Steps:
1; 2 As shown in FIG. 1, the chemical synthesis method of 3-chloro-1-propanol specifically includes the following steps
(1) Put 440kg of 1,3-propanediol, 600kg of hydrochloric acid, and 3kg of benzenesulfonic acid into a 2000L glass-lined kettle with a stirring and reflux condensing device, heat up to 90 ° C with stirring, and keep the reaction warm;(2) After holding for 3 hours, lower the temperature of the kettle to 50 ° C, then put 460kg of hydrochloric acid into the kettle, continue to warm to 90 ° C and keep the reaction for 10 hours. The temperature of the kettle is sampled for GC analysis. When the content reaches 80%, the feed liquid is poured into the distillation kettle, and 150 kg of toluene is poured into the distillation kettle to carry out the reaction of heating and refluxing with water;(3) After the reaction with water is completed, the temperature of the kettle is reduced to room temperature, and sodium bicarbonate is added to neutralize the oil phase in the kettle to neutrality, and the salt formed by the neutralization and excess sodium bicarbonate are filtered;(4) The filtered oil phase is pumped into the rectification kettle, and the solvent toluene is concentrated under normal pressure. After the toluene is collected, the product is rectified by high vacuum. The rectified fraction is put into the reaction kettle to continue the reaction.The content detected by GC gas chromatography was 99.3%, and the product yield was 96%.
References:
CN110668918,2020,A Location in patent:Paragraph 0030-0043
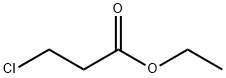
623-71-2
158 suppliers
$27.57/250mgs:

627-30-5
319 suppliers
$9.00/1g
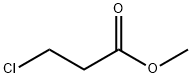
6001-87-2
82 suppliers
$45.00/25mg

627-30-5
319 suppliers
$9.00/1g

29560-84-7
10 suppliers
inquiry

627-30-5
319 suppliers
$9.00/1g
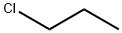
540-54-5
215 suppliers
$19.00/25mL
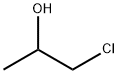
127-00-4
135 suppliers
$28.00/25mL

627-30-5
319 suppliers
$9.00/1g