
1,3-Propanediol synthesis
- Product Name:1,3-Propanediol
- CAS Number:504-63-2
- Molecular formula:C3H8O2
- Molecular Weight:76.09
CH2CHCHO + H2O → HOHCH2CH2CHO
HOHCH2CH2CHO + H2 → HOHCH2CH2CH2OH
The addition of water under mild acidic conditions gives 3-hydroxypropionaldehyde with high selectivity. Preferentially buffer solutions with a pH 4-5 or weak acidic ion exchange resins are used as catalysts. Further hydrogenation of this aqueous solutions gives 1,3-propanediol. There is an alternative route via hydroformylation of ethylene oxide and subsequent hydrogenation of the intermediate 3-hydroxypropionaldehyde.
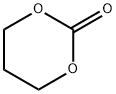
2453-03-4
125 suppliers
$40.00/1g

504-63-2
559 suppliers
$9.00/5g
Yield:504-63-2 99%
Reaction Conditions:
with [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II);potassium tert-butylate;hydrogen in tetrahydrofuran at 140; under 38002.6 Torr; for 2 h;Autoclave;Pressure;
Steps:
21 Example 21: Hydrogenation of cyclic carbonate, 1,3-dioxan-2-one catalyzed by ruthenium complex 1a
In a glove box, into a 125 mL autoclave was charged with ruthenium complex 1a (8.7 mg, 0.0143 mmol),potassium tert-butoxide (1.6 mg, 0.0143 mmol), tetrahydrofuran (20 mL) and 1,3-dioxan-2-one (2.92 g, 28.6 mmol). Theautoclave was sealed, removed from the glove box, and filled with hydrogen gas to 50 atm. The reaction vessel washeated in an oil bath at 140°C with stir for 2 hours. The reaction vessel was cooled in an ice-water bath for 1.5 hours,and the excess of hydrogen was slowly deflated. The conversion rate for the reaction was determined as >99% with pxyleneas internal standard by using gas chromatography. The yields of methanol and diol are 99% and 99%, respectively
References:
EP2910540,2015,A1 Location in patent:Paragraph 0097

36678-05-4
14 suppliers
inquiry

504-63-2
559 suppliers
$9.00/5g
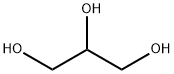
56-81-5
1699 suppliers
$5.00/25g

504-63-2
559 suppliers
$9.00/5g
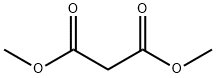
108-59-8
631 suppliers
$5.00/25g

504-63-2
559 suppliers
$9.00/5g
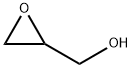
556-52-5
285 suppliers
$17.67/1gm:

504-63-2
559 suppliers
$9.00/5g