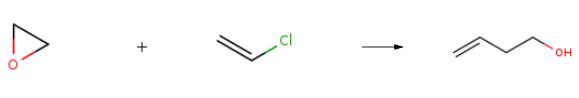
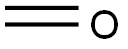3-Buten-1-ol synthesis
- Product Name:3-Buten-1-ol
- CAS Number:627-27-0
- Molecular formula:C4H8O
- Molecular Weight:72.11
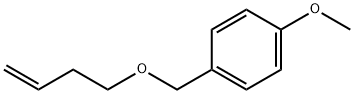
142860-83-1
3 suppliers
inquiry

627-27-0
405 suppliers
$8.00/1g
Yield:627-27-0 93%
Reaction Conditions:
with t-butyl bromide in acetonitrile; for 1 h;Reflux;chemoselective reaction;
Steps:
Representative procedure for the deprotection of the PMB ethers by t-BuBr
General procedure: To asolution of the PMB ether (1 mmol) in acetonitrile (10 mL), t-BuBr (1.1 equiv)was added and stirred at reflux. After completion of the reaction (monitored byTLC), it was concentrated under reduced pressure and the resulting crude wasdissolved in ethyl acetate (50 mL) and washed with saturated sodiumhydrogenocarbonate (25 mL). The aqueous layer was extracted with ethylacetate (2 5 mL) and the combine organic layer was washed with brinesolution, dried (MgSO4), concentrated under reduced pressure and the residuewas purified by column chromatography (silica gel, EtOAc, cyclohexane) toafford the corresponding alcohol.
References:
Rival, Nicolas;Albornoz Grados, Arantxa;Schiavo, Lucie;Colobert, Fran?oise;Hanquet, Gilles [Tetrahedron Letters,2015,vol. 56,# 49,p. 6823 - 6826]

927-74-2
428 suppliers
$6.00/5g

627-27-0
405 suppliers
$8.00/1g
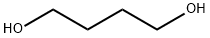
110-63-4
705 suppliers
$24.80/250g
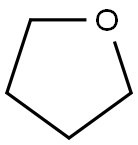
109-99-9
1058 suppliers
$9.00/5ml

627-27-0
405 suppliers
$8.00/1g