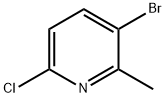
3-Bromo-6-chloro-2-methylpyridine synthesis
- Product Name:3-Bromo-6-chloro-2-methylpyridine
- CAS Number:132606-40-7
- Molecular formula:C6H5BrClN
- Molecular Weight:206.47
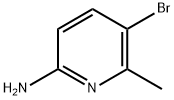
42753-71-9
394 suppliers
$6.00/5g

132606-40-7
234 suppliers
$5.00/1g
Yield: 70%
Reaction Conditions:
Stage #1:5-bromo-6-methyl-pyridin-2-ylamine with hydrogenchloride;pyridine hydrochloride;copper(l) chloride;sodium nitrite in dichloromethane;water at 0 - 20; for 1.5 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water
Steps:
To a 2L 3-neck RBF was added CH2Cl2 (900 mL) followed by 2-amino-6-methyl- 5-bromopyridine (74.23 g, 0.39 mol), pyridine-HCl (139 g, 1.2 mol), NaNO2 (83.26 g, 1.2 mol) and CuCl (3.76 g, 5% w/w to starting material). The mixture was cooled to 0-10 0C in an ice- water bath and cone. HCl (4.5 mL, 6% v/w to starting material) was added dropwise and the mixture stirred at 0-10 0C for 30 min. The cooling bath was removed and the mixture was stirred at rt for Ih. The reaction mixture was quenched with saturated aqueous NaHCO3 (400 mL), the layers separated and the aqueous layer was extracted with CH2Cl2 (100 mL). The combined organic layers were concentrated to dryness and hexanes (750 mL) was added to the residue under stirring. The solid was filtered and washed with hexane and the filtrate was concentrated to dryness to afford pure product as a light yellow crystalline solid (61 g, 70%).
References:
ANORMED INC. WO2007/22371, 2007, A2 Location in patent:Page/Page column 49
126717-59-7
240 suppliers
$6.00/1g

132606-40-7
234 suppliers
$5.00/1g
22280-60-0
328 suppliers
$10.00/1g

132606-40-7
234 suppliers
$5.00/1g
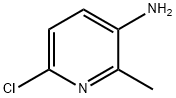
164666-68-6
236 suppliers
$9.00/1g

132606-40-7
234 suppliers
$5.00/1g