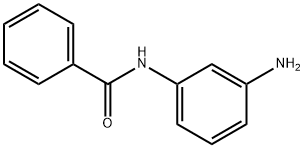
3'-Aminobenzanilide synthesis
- Product Name:3'-Aminobenzanilide
- CAS Number:16091-26-2
- Molecular formula:C13H12N2O
- Molecular Weight:212.25
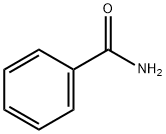
55-21-0
408 suppliers
$5.00/5 g
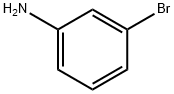
591-19-5
333 suppliers
$10.00/10g

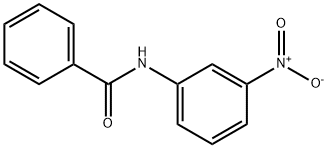
16091-26-2
123 suppliers
$20.00/1 g
Yield: 92%
Reaction Conditions:
with copper(l) iodide;dimethylaminoacetic acid;potassium carbonate in N,N-dimethyl-formamide at 100; for 24 h;Schlenk technique;Inert atmosphere;Goldberg Reaction;
Steps:
3.3. General Procedure for the Coupling of Aryl Bromides with Amides Using Copper/N,N-DimethylglycineCatalytic System
General procedure: A Schlenk tube was charged with amide (1.2 mmol), aryl halide (1 mmol), CuI (0.05 or 0.1 mmol), N,N-dimethylglycine (0.1 or 0.2 mmol), and potassium carbonate (2 mmol). The tube was evacuated and backfilled with argon at room temperature. DMF (0.5 mL) was added under argon via syringe. The Schlenk tube was immersed in a preheated oil bath and the reaction mixture was stirred for the specified time at the indicated temperature. The cooled mixture was partitioned between water and ethyl acetate. The organic layer was separated and the aqueous layer was extracted with ethyl acetate.The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (eluting with 1:8 to 1:2 ethyl acetate/petroleum ether) to give the the desired N-aryl amides.
References:
Jiang, Liqin [Molecules,2014,vol. 19,# 9,p. 13448 - 13460] Location in patent:supporting information
4771-08-8
31 suppliers
$15.00/5mg

16091-26-2
123 suppliers
$20.00/1 g
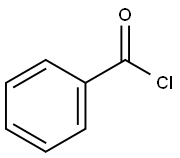
98-88-4
597 suppliers
$10.00/5g

16091-26-2
123 suppliers
$20.00/1 g
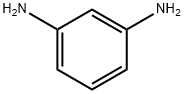
108-45-2
477 suppliers
$13.00/25g

98-88-4
597 suppliers
$10.00/5g

16091-26-2
123 suppliers
$20.00/1 g
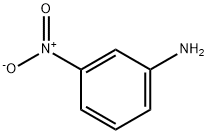
99-09-2
20 suppliers
$14.00/1g

16091-26-2
123 suppliers
$20.00/1 g