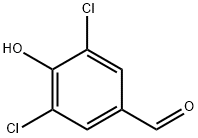
3,5-DICHLORO-4-HYDROXYBENZALDEHYDE synthesis
- Product Name:3,5-DICHLORO-4-HYDROXYBENZALDEHYDE
- CAS Number:2314-36-5
- Molecular formula:C7H4Cl2O2
- Molecular Weight:191.01
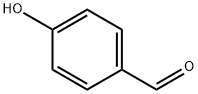
123-08-0
938 suppliers
$5.00/10g

2314-36-5
122 suppliers
$12.00/250mg
Yield: 97.4%
Reaction Conditions:
with N-chloro-N-(benzenesulfonyl)benzenesulfonamide in acetonitrile at 20 - 25; for 0.666667 h;Green chemistry;
Steps:
General procedure for the dichlorination reaction
General procedure: To a solution of 4-nitroaniline, 1b (0.99 g, 7.2 mmol) in 7 mL specially dried MeCN was added N-chloro-N-(phenylsulfonyl)benzene sulfonamide (4.8 g, 14.4 mmol) in a 25 mL round bottom flask. The reaction mixture was stirred for 10-15 minutes at 20-25 °C, monitored by GC. After completion of the reaction, MeCN was distilled under vacuum at 40-45 °C. The residue was treated with 20 mL mixture of MDC and water, stirred the mixture for 10-15 minutes; MDC layer was separated and washed with 5 % sodium bicarbonate solution, after separation MDC layer was dried over sodium sulfate and evaporated under vacuum to obtain 2,6-dichloro-4-nitroaniline, 2b produced 1.45 g (99.0 % yield, 99.2 % purity) as a yellow powder.
References:
Misal, Balu;Palav, Amey;Ganwir, Prerna;Chaturbhuj, Ganesh [Tetrahedron Letters,2021,vol. 63,art. no. 152689] Location in patent:supporting information
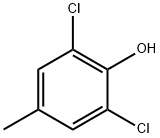
2432-12-4
94 suppliers
$27.00/1g

2314-36-5
122 suppliers
$12.00/250mg
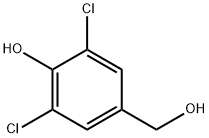
22002-17-1
24 suppliers
$432.00/1g

2314-36-5
122 suppliers
$12.00/250mg
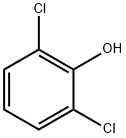
87-65-0
406 suppliers
$10.00/10 g
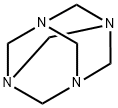
100-97-0
7 suppliers
$14.00/250G

2314-36-5
122 suppliers
$12.00/250mg

87-65-0
406 suppliers
$10.00/10 g

67-66-3
6 suppliers
$33.60/500ml

2314-36-5
122 suppliers
$12.00/250mg