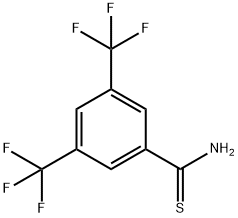
3,5-DI(TRIFLUOROMETHYL)BENZENE-1-CARBOTHIOAMIDE synthesis
- Product Name:3,5-DI(TRIFLUOROMETHYL)BENZENE-1-CARBOTHIOAMIDE
- CAS Number:317319-15-6
- Molecular formula:C9H5F6NS
- Molecular Weight:273.2
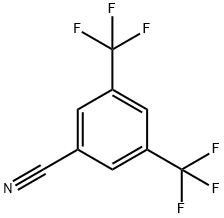
27126-93-8
283 suppliers
$6.00/1g

317319-15-6
50 suppliers
$51.39/250mg
Yield: 98%
Reaction Conditions:
Stage #1:3,5-bis(trifluoromethyl)benzonitrile with sodium hydrogen sulfide monohydrate in N,N-dimethyl-formamide at 10; for 0.416667 h;Inert atmosphere;Large scale;
Stage #2: with magnesium(II) chloride hexahydrate in N,N-dimethyl-formamide at 10; for 0.833333 h;Large scale;Reagent/catalyst;Temperature;Time;
Steps:
26 Synthesis of 3,5-bis(trifluoromethyl)benzothioamide:
Synthesis of 3,5-bis(trifluoromethyl)benzothioamide: A 20-L, multi-necked flask equipped with an over-head stirrer, and thermometer socket was charged with bis(trifluoromethyl)benzonitrile (1.25 kg, 1.0 equiv.) and DMF (6.25 L, 5V), and the resulting mixture was stirred under nitrogen at room temperature (28 ° C). The reaction mixture was cooled to 10 °C and 0.775 g NaSH.H20 (2 equiv.) was added over a period of 10 mins. After stirring for 15 minutes, MgCl2.6H20 (1.169 kg, 1.1 equiv.) was added portionwise over a period of 15 minutes and the reaction was stirred for another 35 minutes. The progress of the reaction (green-colored solution) was monitored by HPLC which showed 99.6% product and 0.03% benzonitrile. The reaction mixture was cooled to 0- 5 °C and 30% dil. HC1 (3.75 L) was added dropwise to adjust the pH to 2-3. The resulting mass was extracted with MTBE (5 L x 1). The layers were separated and 1 L of DM water was added to the aqueous layer, which was re-extracted with MTBE (2.5 L x 1). The combined organic layers were washed with brine (4.5 L x 3), dried and concentrated under vacuum. Hexane was added to the solid obtained, chased and the product was isolated as yellow solid (1.400 Kg, 98.0 %). Purity: 99.28% (HPLC). 1H NMR (300 MHz, CDC13) δ:8.27 (s, 1H), 8.53(s, 2H), 10.0 (s, 1H), 10.38 (s, 1H).
References:
KARYOPHARM THERAPEUTICS, INC.;SANDANAYAKA, Vincent, P.;SHACHAM, Sharon;MCCAULEY, Dilara;SHECHTER, Sharon WO2013/19548, 2013, A1 Location in patent:Page/Page column 83-84