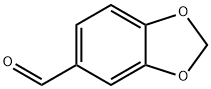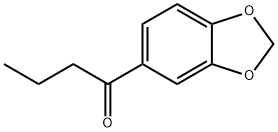
3,4-(METHYLENEDIOXY)BUTYROPHENONE synthesis
- Product Name:3,4-(METHYLENEDIOXY)BUTYROPHENONE
- CAS Number:63740-97-6
- Molecular formula:C11H12O3
- Molecular Weight:192.21
Yield:63740-97-6 75 g
Reaction Conditions:
with zinc(II) oxide;zinc(II) chloride in dichloromethane at 0 - 5; for 5 h;
Steps:
7 Preparation of 5-butyl-l,3-Benzodioxole (using 0.5 moles of Zinc oxide and 0.05 moles of Zinc chloride per mole of acyl chloride using dichloromethane as solvent)
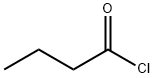
122 g of 1,3-benzodioxole and 190 g of dichloromethane were charged into a 1 litre reaction flask and the mixture was cooled to 0°C under stirring. 41 g of Zinc oxide and 7 g of Zinc chloride were added under stirring. Subsequently, 106.5 g of butanoyl chloride was added to the above mixture in 4 hours maintaining the temperature of the reaction medium between 0°C and 5°C under stirring. The reaction medium was stirred for another 1 hour until the acylation reaction was substantially completed. The reaction mass was subjected to aqueous workup to remove Zinc chloride and butanoic acid and the organic layer was separated and distilled to recover 65 g of unreacted 1,3-benzodioxole and 75 g of l-(l,3-benzodioxol-5-yl)-l-butanone with a GC purity of > 99%. The l-(l,3-benzodioxol-5-yl)-l-butanone was subjected to selective reduction and dehydration as discussed in Example 2 to obtain 66 g of 5-butyl-l,3-benzodioxole with a yield of 115.8% (wt./wt. on 1,3-benzodioxole consumed) and purity of > 99% by GC analysis.
References:
ANTHEA AROMATICS PRIVATE LIMITED;MOHAPATRA, Manoj Kumar;BENDAPUDI, Ramamohanrao;MENACHERRY, Paul Vincent;PAUL, Vincent WO2018/150230, 2018, A1 Location in patent:Paragraph 0077; 0078
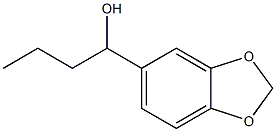
6890-31-9
4 suppliers
inquiry

63740-97-6
89 suppliers
$8.00/1g
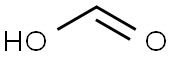
64-18-6
1112 suppliers
$22.12/250ML
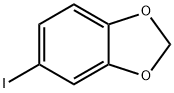
5876-51-7
118 suppliers
$57.00/250mg

106-94-5
580 suppliers
$10.00/5 mL

63740-97-6
89 suppliers
$8.00/1g