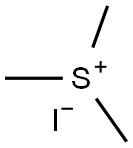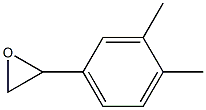
(3,4-dimethyl-phenyl)-oxirane synthesis
- Product Name:(3,4-dimethyl-phenyl)-oxirane
- CAS Number:141303-37-9
- Molecular formula:C10H12O
- Molecular Weight:148.2017
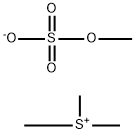
5973-71-7
338 suppliers
$10.00/25g
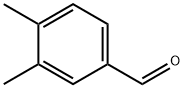
2181-44-4
55 suppliers
$40.00/25g

141303-37-9
0 suppliers
inquiry
Yield:141303-37-9 78%
Reaction Conditions:
with sodium hydroxide;tetrabutylammomium bromide in dichloromethane;water;
Steps:
Preparation of 3,4-Dimethylphenyloxirane
Preparation of 3,4-Dimethylphenyloxirane The procedure was based on methods described by Brandes and Jacobsen, (Tetrahedron Asym. 8:3927, 1997); and Kaufman (Syn. Commun. 23:473, 1993). A solution of trimethylsulfonium methylsulfate (3.95 g, 0.021 mol) in 8 mL water was added slowly to a biphasic mixture of 50% NaOH (20 mL), 3,4-dimethyl-benzaldehyde (1.34 g, 0.01 mmol), tetrabutylammonium bromide (0.025 g, 0.0782 mmol), and CH2Cl2 (26 mL). The reaction was heated at 50° C. for 13 hours and then cooled to room temperature. The reaction was diluted carefully with brine (50 mL) and diethyl ether (3*70 mL), then filtered to remove the solids. The aqueous layer was extracted with diethyl ether (3*70 mL), and the combined organic layers were washed with brine (50 mL) and dried over Na2SO4, filtered, and concentrated in vacuo to afford the product 3,4-dimethylphenyl oxirane as a light yellow oil (1.15 g, yield 78%). TLC Rf=0.9 (1:2 EtOAc/Hexane); 1H NMR (300 MHz, CDCl3) δ 7.39 (s, 1H), 7.21 (d, 1H), 7.19 (s, 1H), 3.80 (m, 1H), 3.17 (m, 1H), 2.80 (m, 1H), 2.23 (s, 6H).
References:
US2003/78260,2003,A1