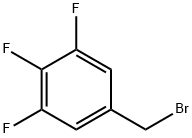
3,4,5-TRIFLUOROBENZYL BROMIDE synthesis
- Product Name:3,4,5-TRIFLUOROBENZYL BROMIDE
- CAS Number:220141-72-0
- Molecular formula:C7H4BrF3
- Molecular Weight:225.01
220227-37-2
109 suppliers
$15.00/1 g

220141-72-0
139 suppliers
$13.00/1g
Yield:-
Reaction Conditions:
with dibromo sulfoxide in dichloromethane at 20; for 16 h;
Steps:
62.B
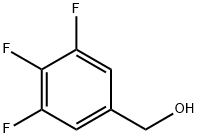
To a solution of the (3,4,5-Trifluorophenyl)methanol (40 mmol) in CH2Cl2 (150 ml), a solution of thionyl bromide (6.16 ml, 80 mmol) in CH2Cl2 (50 ml) was added slowly. The reaction mixture stirred at room temperature for 16h and poured into ice-water (200 ml). The organic layer was separated and washed with saturated NaHCO3 (2x200 ml), water (200 ml), dried (MgSO4) and evaporated to obtain the corresponding bromo compound as a pale yellow oil in quantitative yield. The crude product was carried forward for the next reaction without further purification.
References:
VALEANT RESEARCH & DEVELOPMENT WO2007/14011, 2007, A2 Location in patent:Page/Page column 91