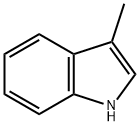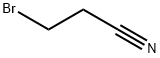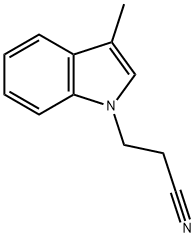
3-(3-METHYL-1H-INDOL-1-YL)PROPANENITRILE synthesis
- Product Name:3-(3-METHYL-1H-INDOL-1-YL)PROPANENITRILE
- CAS Number:4414-81-7
- Molecular formula:C12H12N2
- Molecular Weight:184.24
Yield:4414-81-7 74%
Reaction Conditions:
Stage #1: 3-Methylindolewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.75 h;
Stage #2: bromopropionitrile in N,N-dimethyl-formamide at 20; for 6 h;
Steps:
General Procedure A: alkylation of 1 //-indole.
General procedure: In a 50 mL oven-dried round bottom flask, l/Z-indoles (1.71 mmol) was dissolved in 20 mL anhydrous DMF and cooled down to 0 °C. NaH (0.10 g, 2.57 mmol) was added portionwise. The resulting heterogeneous mixture was stirred at 0 °C for 45 min. 3-Bromopropanenitrile (0.21 mL, 2.57 mmol) was then added dropwise via syringe and the reaction mixture was allowed to warm up to room temperature. After 6 h, the reaction was quenched with 1M HC1, extracted two times with ethyl acetate and concentrated under vacuum. The crude product was purified via flash column chromatography to offer the desired product.
References:
WO2019/191410,2019,A1 Location in patent:Page/Page column 31; 35
![2-Butenenitrile, 4-[(2-chlorophenyl)(2-cyanoethyl)amino]-, (Z)- (9CI)](/CAS/20210305/GIF/101195-43-1.gif)
101195-43-1
0 suppliers
inquiry

4414-81-7
22 suppliers
inquiry
101195-51-1
1 suppliers
inquiry
101195-52-2
0 suppliers
inquiry
![2-Butenenitrile, 4-[(2-chlorophenyl)(2-cyanoethyl)amino]-, (E)- (9CI)](/CAS/20210305/GIF/101195-42-0.gif)
101195-42-0
0 suppliers
inquiry

4414-81-7
22 suppliers
inquiry

101195-51-1
1 suppliers
inquiry

101195-52-2
0 suppliers
inquiry
![3-[(2-Chlorophenyl)amino]propanenitrile](/CAS/GIF/94-89-3.gif)
94-89-3
45 suppliers
$63.00/50mg

4414-81-7
22 suppliers
inquiry