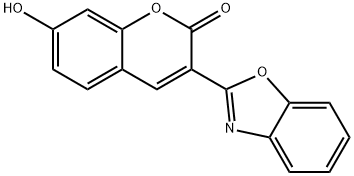
3-(2-BENZOXAZOLYL)-7-HYDROXYCOUMARIN synthesis
- Product Name:3-(2-BENZOXAZOLYL)-7-HYDROXYCOUMARIN
- CAS Number:64887-40-7
- Molecular formula:C16H9NO4
- Molecular Weight:279.25
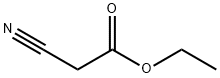
105-56-6
442 suppliers
$10.00/5ml
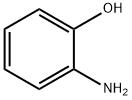
95-55-6
543 suppliers
$5.00/5G
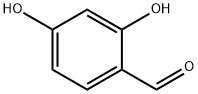
95-01-2
495 suppliers
$10.00/1g

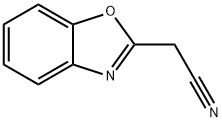
64887-40-7
14 suppliers
inquiry
Yield:64887-40-7 87%
Reaction Conditions:
with acetic acid in glycerol at 80; for 14 h;Schlenk technique;Green chemistry;
Steps:
Typical experimental procedure for synthesis of 3-(benzo[d]oxazol-2-yl)-2H-chromen-2-one
General procedure: The main synthetic root is described in Scheme 1. A mixture of salicylaldehyde(0.50 mmol), o-aminophenol (0.60 mmol) and ethyl cyanoacetate (0.55 mmol) wasplaced in a 10-mL Schlenk tube, and then, 1 mmol acetic acid and 0.2 mL glycerolwere added. The reaction mixture was stirred at the indicated temperature for 14 h.After completion of the reaction, a solution of Na2CO3and ethanol were added to themixture and vigorously stirred for a while. Finally, the precipitate was filtrated andwashed with 50% ethanol without further purification to afford the corresponding pure compound
References:
Gao, Jiali;Liu, Ao;Li, Minghang;Wang, Yuying;Xiao, Yudi;Lü, Chengwei;An, Yue [Research on Chemical Intermediates,2021,vol. 47,# 8,p. 3179 - 3187]
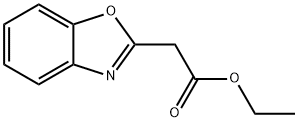
16105-44-5
34 suppliers
$31.00/100mg

95-01-2
495 suppliers
$10.00/1g

64887-40-7
14 suppliers
inquiry