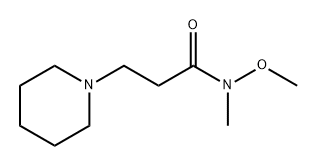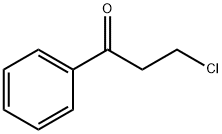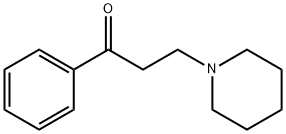
3-(1-Piperidinyl)propiophenone HCl synthesis
- Product Name:3-(1-Piperidinyl)propiophenone HCl
- CAS Number:73-63-2
- Molecular formula:C14H19NO
- Molecular Weight:217.31
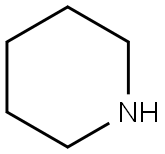
110-89-4
0 suppliers
$15.96/100ML
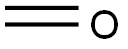
50-00-0
894 suppliers
$10.00/25g
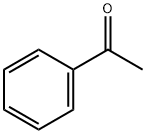
98-86-2
794 suppliers
$9.00/5g

73-63-2
56 suppliers
$95.00/100mg
Yield:73-63-2 96%
Reaction Conditions:
with C7H13N2O3S(1+)*C10H15O4S(1-) at 25; for 0.5 h;Mannich Aminomethylation;
Steps:
2.3. Mannich reaction: a typical procedure
General procedure: In a classic tryout, aniline (0.9 mL, 10 mmol, 1 equiv.), benzaldehyde (1 mL, 10 mmol, 1 equiv.), cyclohexanone/acetophenone (10 mmol, 1 equiv.) and stoichiometric amount of catalyst were placed in a round bottom flask with a magnetic stirrer at a constant temperature water bath at 25°C (Scheme 2 ) [30,31]. The reaction mixture turned turbid within minutes and progress of reaction was continuously monitored by TLC. After completion of reaction, as indicated by TLC, the IL was separated from the product by adding 3-5mL of water. The solid product was filtered, dried and then recrystallized using acetonitrile and further dried in vacuo until constant weight was obtained. Finally the water layer containing IL was concentrated using rotary evaporator. After washing with diethylether and ethyl acetate, IL was then vacuum dried at 70°C for 12 h and was reused for next cycle.
References:
Hamzah, Ahmad Sazali;Jabeen, Erum;Leveque, Jean-Marc;Sardar, Sabahat;Wilfred, Cecilia Devi [Journal of Molecular Liquids,2020,vol. 320,art. no. 114370]
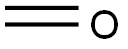
50-00-0
894 suppliers
$10.00/25g
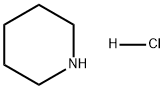
6091-44-7
0 suppliers
$59.21/100g

98-86-2
794 suppliers
$9.00/5g

73-63-2
56 suppliers
$95.00/100mg
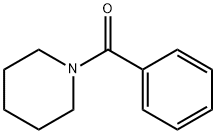
776-75-0
115 suppliers
$15.00/250mg
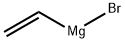
1826-67-1
220 suppliers
$60.89/100ml

73-63-2
56 suppliers
$95.00/100mg