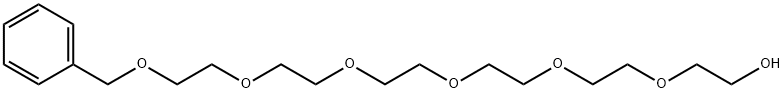
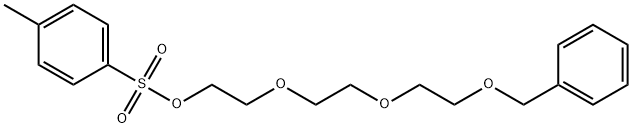
2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL synthesis
- Product Name:2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL
- CAS Number:24342-68-5
- Molecular formula:C19H32O7
- Molecular Weight:372.45

2615-15-8
234 suppliers
$6.00/1g
100-39-0
439 suppliers
$10.00/10 g
![2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/24342-68-5.gif)
24342-68-5
119 suppliers
$17.00/100mg
96922-86-0
1 suppliers
inquiry
Yield: 20%
Reaction Conditions:
in aqueous sodium hydroxide at 100; for 2 h;
Steps:
10.a (E)-4-(1,3-Bis(cyclohexylmethyl)-1,2,3,6-tetrahydro-2,6-dioxo-9H-purin-8-yl)cinnamic Acid Nonaethylene Glycol Methyl Ether Ester
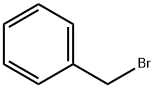
(a) nonaethylene Glycol Monomethyl Ether A mixture of hexaethylene glycol (Aldrich, 100 g) and benzyl bromide (Aldrich, 12 g) in aqueous sodium hydroxide (50% (w/w), 80 ml) was stirred at 100° C. (oil bath) under nitrogen for 2 h. The reaction mixture was then cooled to ambient temperature, diluted with water to a total volume of 500 ml, and extracted with diethyl ether (200 ml) to remove dibenzylated product. sodium chloride (100 g) was added to the aqueous layer, which was extracted further with diethyl ether (6*100 ml). These ether extracts were combined, dried (sodium sulfate), and concentrated to give hexaethylene glycol monobenzyl ether as an oil (25 g, 20% based on glycol), 1H-NMR (CDCl3) δ: 7.30 (m, 5, 5 phenyl CH), 4.53 (s, 2, benzyl CH2), 3.69-3.54 (m, 22, 11 OCH2), 3.06 (br s, 3, OH and CH2O).
References:
Glaxo Wellcome Inc. US6355646, 2002, B1 Location in patent:Page column 13

2615-15-8
234 suppliers
$6.00/1g
100-44-7
660 suppliers
$13.50/250G
![2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/24342-68-5.gif)
24342-68-5
119 suppliers
$17.00/100mg

2615-15-8
234 suppliers
$6.00/1g

100-39-0
439 suppliers
$10.00/10 g
![2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/24342-68-5.gif)
24342-68-5
119 suppliers
$17.00/100mg
84131-04-4
35 suppliers
inquiry
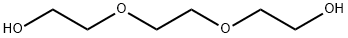
112-27-6
772 suppliers
$6.00/100g
![2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/24342-68-5.gif)
24342-68-5
119 suppliers
$17.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2-[2-[2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/24342-68-5.gif)
24342-68-5
119 suppliers
$17.00/100mg