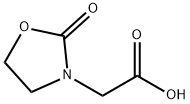
(2-OXO-1,3-OXAZOLIDIN-3-YL)ACTETIC ACID synthesis
- Product Name:(2-OXO-1,3-OXAZOLIDIN-3-YL)ACTETIC ACID
- CAS Number:75125-23-4
- Molecular formula:C5H7NO4
- Molecular Weight:145.11
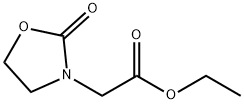
75125-24-5
13 suppliers
inquiry

75125-23-4
29 suppliers
$130.00/500mg
Yield:75125-23-4 89.5%
Reaction Conditions:
with hydrogenchloride in water at 20;
Steps:
5.B Step B: 2-(2-oxooxazolidin-3-yl)acetic acid.
A mixture of ethyl 2-(2-oxooxazolidin-3-yl)acetate (200 mg, 1.16 mmol) in cone. HCI (2 mL) was stirred at room temperature (r.t.) overnight. The mixture was concentrated and the residue was dissolved in acetone and dried over anhydrous a2S04. The acetone was evaporated to give the desired compound (150 mg, 89.5% yield). MS: 146.0 (M+l)+.
References:
WO2013/107405,2013,A1 Location in patent:Page/Page column 66

3356-88-5
55 suppliers
$60.00/500mg

75125-23-4
29 suppliers
$130.00/500mg
![Glycine, N-[(2-bromoethoxy)carbonyl]-, methyl ester](/CAS/20210305/GIF/65538-58-1.gif)
65538-58-1
0 suppliers
inquiry

75125-23-4
29 suppliers
$130.00/500mg
