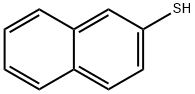
2-Naphthalenethiol synthesis
- Product Name:2-Naphthalenethiol
- CAS Number:91-60-1
- Molecular formula:C10H8S
- Molecular Weight:160.24
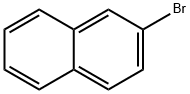
580-13-2
509 suppliers
$6.00/1g

91-60-1
222 suppliers
$15.00/1g
Yield:91-60-1 95%
Reaction Conditions:
Stage #1: 2-bromonaphthalenewith water;caesium carbonate;sodium thiosulfate;bis(dibenzylideneacetone)-palladium(0);XPhos in toluene;tert-butyl alcohol at 80; for 24 h;Inert atmosphere;
Stage #2: with hydrogenchloride;zinc in water; for 1 h;Cooling with ice;
Steps:
2.1. General procedure for Tables 1 and 2
An oven-dried Schlenk tube was charged with aryl halides 1 (0.5 mmol), sodium thiosulfate 2 (100 mg), Cs2CO3 (1 mmol, 325 mg), appropriate amount of Pd2(dba)3/Pd(OAc)2 and Xphos, equipped with a magnetic stir bar. The tube was evacuated and backfilled with argon three times before the solvent was added. The mixture was stirred until homodisperse at rt. Then the tube was stirred at 80 °C for 24 h. The solid substance was separated from the reaction mixture and washed with ether. Zn dust (0.5 g) and HCl (10%, 5 mL) was added to the solid substance with cooling by ice-water. After stirred for 1 h, the mixture was extracted with ethyl acetate. The organic layer was washed with H2O and brine and dried over Na2SO4. Removal of solvent in vacuo provided the desired product with satisfactory purity. Some relatively stable products were purified via column chromatography.
References:
Yi, Jun;Fu, Yao;Xiao, Bin;Cui, Wei-Chen;Guo, Qing-Xiang [Tetrahedron Letters,2011,vol. 52,# 2,p. 205 - 208] Location in patent:experimental part
93-11-8
238 suppliers
$14.00/5g

91-60-1
222 suppliers
$15.00/1g
3857-83-8
73 suppliers
$13.50/250mg

91-60-1
222 suppliers
$15.00/1g
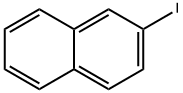
612-55-5
113 suppliers
$48.00/500mg

91-60-1
222 suppliers
$15.00/1g
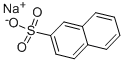
532-02-5
356 suppliers
$10.00/10g

91-60-1
222 suppliers
$15.00/1g