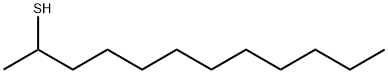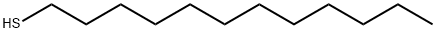
1-Dodecanethiol synthesis
- Product Name:1-Dodecanethiol
- CAS Number:112-55-0
- Molecular formula:C12H26S
- Molecular Weight:202.4

112-52-7
287 suppliers
$7.00/25g
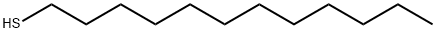
112-55-0
329 suppliers
$14.00/5mL
Yield:112-55-0 244 g
Reaction Conditions:
with sodium hydrogensulfide;tetrabutylammomium bromide in water;chlorobenzene at 65 - 70; for 12 h;
Steps:
5 Example 5
The second phase (445 g) is distilled to obtain n-chlorododecane (V) (143 g). 143 g of the n-chlorododecane (V), 100 g of chlorobenzene, 180 g of a 32% by weight sodium bisulfide solution in water and 4 g of tetra-butylammonium bromide are added into a 1000 ml four-necked flask with a stirrer, a thermometer and a condenser. At a temperature in the range of from 65 to 70° C., the mixture is reacted for 12 h. The mixture is separated into an aqueous layer and an organic layer. The organic layer is n-dodecanethiol (III) (244 g).
References:
US2015/336910,2015,A1 Location in patent:Paragraph 0277
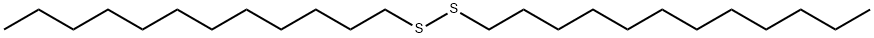
2757-37-1
54 suppliers
inquiry
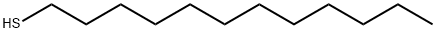
112-55-0
329 suppliers
$14.00/5mL
![Benzene, 1-[[(1Z)-2-(dodecylthio)ethenyl]sulfonyl]-4-methyl-](/CAS/20210305/GIF/607731-47-5.gif)
607731-47-5
0 suppliers
inquiry
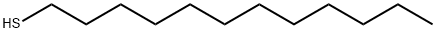
112-55-0
329 suppliers
$14.00/5mL