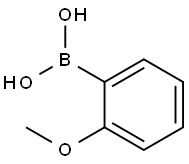
2-Methoxyphenylboronic acid synthesis
- Product Name:2-Methoxyphenylboronic acid
- CAS Number:5720-06-9
- Molecular formula:C7H9BO3
- Molecular Weight:151.96
Yield:5720-06-9 69%
Reaction Conditions:
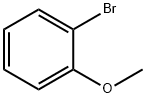
Stage #1: 2-bromoanisolewith magnesium in tetrahydrofuran at 20; for 1 h;Heating;
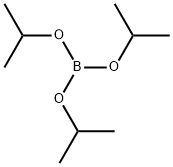
Stage #2: Triisopropyl borate in tetrahydrofuran at -78 - 20; for 18 h;
Stage #3: with water in tetrahydrofuran; for 1 h;
Steps:
3.4.1 Method 1
Magnesium (5.30 g, 0.22 mol), was activated by heating and stirring under nitrogen for 1 h at 90 °C after which time it was allowed to cool to room temperature and dry THF (30 mL) was added. A solution of 2-bromoanisole (5.0 mL, 42 mmol) in dry THF (50 mL) was added slowly via syringe to give a black suspension. The solution was allowed to stir for 1 h at room temperature. The reaction mixture was then added slowly, via cannula filtration, to a solution of triisopropylborate (15 mL, 43 mmol) in THF (15 mL) at 78 °C. The reaction mixture was allowed to warm to room temperature while stirring overnight (18 h). Water (60 mL) was added and the suspension was allowed to stir for a further 1 h. The reaction mixture was evaporated in vacuo and dichloromethane (100 mL) was added. The organic layer was separated, and the aqueous layer was extracted with dichloromethane (4 × 50 mL). The combined organic extracts were dried over MgSO4 and filtered. The solvent was removed in vacuo to give a light green solid, which was stirred in pentane producing the title compound 14 (4.40 g, 69%) as a white powder. m.p. 109-111°C; 1H NMR (300MHz; CDCl3) δ=7.87 (dd, 1H, J1=7.4Hz, J2=1.9Hz), 7.45 (ddd, 1H, J1=11.7Hz, J2=4.5Hz, J3=2.5Hz), 7.03 (app t, 1H, J=7.2Hz), 6.91 (d, 1H, J=8.4Hz), 6.63 (s, 2H, B(OH)2), 3.90 (s, 3H, OCH3); 13C NMR (75MHz; CDCl3) 164.5 (4°), 136.9, 132.9, 121.3, 110.0, 55.5 (OMe); IR (KBr) νmax 3384, 1601, 1412, 1229, 1164, 1055, 1024, 756, and 658cm-1.
References:
Sweetman, Brian A.;Guiry, Patrick J. [Tetrahedron,2018,vol. 74,# 38,p. 5567 - 5581]
121-43-7
359 suppliers
$14.00/25mL
578-57-4
300 suppliers
$6.00/10g

5720-06-9
363 suppliers
$5.00/1g

578-57-4
300 suppliers
$6.00/10g

5720-06-9
363 suppliers
$5.00/1g
190788-60-4
108 suppliers
$5.00/250mg

5720-06-9
363 suppliers
$5.00/1g
100-66-3
525 suppliers
$10.00/5G

5720-06-9
363 suppliers
$5.00/1g