
2-(HEXYLOXY)ETHANOL synthesis
- Product Name:2-(HEXYLOXY)ETHANOL
- CAS Number:112-25-4
- Molecular formula:C8H18O2
- Molecular Weight:146.23

111-25-1
323 suppliers
$10.00/5g

107-21-1
1368 suppliers
$10.00/25g

112-25-4
194 suppliers
$11.00/25g
Yield:112-25-4 59%
Reaction Conditions:
Stage #1:ethylene glycol with sodium at 60;Inert atmosphere;
Stage #2:1-bromo-hexane for 3 h;Reflux;Inert atmosphere;
Steps:
2 2.2.1. 2-Alkoxyethanols
General procedure: The following is a general procedure: to a 3-necked round-bottom flask, provided with a refluxcondenser, dropping funnel, and inlet for dry nitrogen was added 50 g (0.8 mol) of dry ethylene glycol. Small pieces of sodium (0.29 mol, 6.8 g), were slowly added to the glycol under vigorous magnetic stirring and the mixture was heated to 60 C until the sodium dissolved. The appropriate 1-bromoalkane (0.25mol for each RBr, 37.76 g; 41.27 g; 48.28 g; 55.30 g for R?C5; C6;C8 and C10, respectively) was added slowly (ca. 30 min) and the solutionwas then kept under reflux for the appropriate time(1 h; 3 h; and 4 h for R?C5; C6; C8 and C10, respectively). The precipitated NaBr was filtered off and the residue was washed with water; the upper layer was separated and dried with anhydrous MgSO4, and the product purified by fractional distillation 2.2.1.2
2-Hexyloxyethanol
Colorless liquid: C6H13OCH2CH2OH; yield: 22 g (59%); b.p.: 111-113 °C/27 torr; d420: 0.8859; 1H NMR: 0.91 (3H, t, J = 8.0, H7), 1.29-1.36 (6H, bs, H6), 1.60 (2H, t, J = 6.0, H5), 3.52 (4H, m, H2, 4), 3.73 (2H, t, J = 6.0, H3), 2.86 (1H, H1).
References:
Loffredo, Carina;Pires, Paulo Augusto R.;Imran, Muhammad;El Seoud, Omar A. [Dyes and Pigments,2013,vol. 96,# 1,p. 16 - 24,9]

107-21-1
1368 suppliers
$10.00/25g

66-25-1
334 suppliers
$16.80/100G

112-25-4
194 suppliers
$11.00/25g
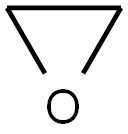
75-21-8
239 suppliers
$33.29/crm44609

111-27-3
536 suppliers
$5.00/25g

112-25-4
194 suppliers
$11.00/25g

107-21-1
1368 suppliers
$10.00/25g

112-25-4
194 suppliers
$11.00/25g