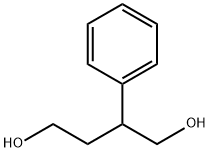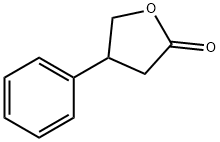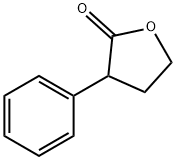
2-FURANONE,4,5-DIHYDRO-3-PHENYL- synthesis
- Product Name:2-FURANONE,4,5-DIHYDRO-3-PHENYL-
- CAS Number:6836-98-2
- Molecular formula:C10H10O2
- Molecular Weight:162.19
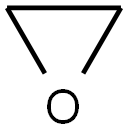
75-21-8
225 suppliers
$39.10/1mL
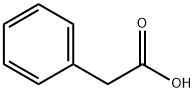
103-82-2
2 suppliers
$22.60/100g

6836-98-2
11 suppliers
$503.99/5MG
Yield:6836-98-2 58%
Reaction Conditions:
Stage #1: phenylacetic acidwith 1,2-diphenyl-1,2-disodiumethane in tetrahydrofuran at 0; for 2 h;Inert atmosphere;
Stage #2: oxirane in tetrahydrofuran at -30; for 0.5 h;Inert atmosphere;
Stage #3: with hydrogenchloride in water;regioselective reaction;
Steps:
4.4. Metalation of arylacetic acids 2, and reaction with electrophiles. General procedure
General procedure: To 5 mL of a 0.24 M solution of diorganometal 1b or 1c (1.2 mmol), chilled at 0 °C, was added a solution of the appropriate arylacetic acid 2 (1.1 mmol) dissolved in 5 mL of dry THF, and the resulting mixture was vigorously stirred for 2 h at 0 °C. To the resulting dark brown mixture, chilled at the same temperature, were added 1.7 mmol of the appropriate electrophile. The resulting mixture was vigorously stirred and allowed to reach rt overnight, after which time it was quenched by slow dropwise addition of H2O (15 mL). The organic solvent was evaporated in vacuo and the resulting mixture was extracted with CH2Cl2 (3×10 mL). The aqueous phase was acidified with 1 N HCl, extracted with CH2Cl2 (3×10 mL), and the organic phases were collected, washed with H2O (1×10 mL), brine (10 mL), dried (Na2SO4), and the solvent was evaporated. Crude reaction products were purified and characterized as reported below. The reaction mixture containing crude β-hydroxyacid 2df was quenched by adding it to 15 mL of 10% HCl containing about 15 g of crushed ice,14 and worked up as described above. After evaporation of the solvent, the resulting crude material was purified and characterized as reported below. Quenching with D2O was realized as described in the above paragraph.
References:
Azzena, Ugo;Dettori, Giovanna;Pisano, Luisa;Pittalis, Mario [Tetrahedron,2011,vol. 67,# 19,p. 3470 - 3475] Location in patent:experimental part
201230-82-2
1 suppliers
inquiry
104-54-1
626 suppliers
$13.00/25g

6836-98-2
11 suppliers
$503.99/5MG
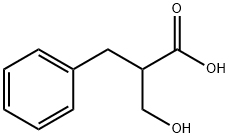
6811-98-9
11 suppliers
inquiry
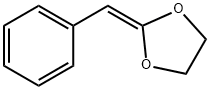
4362-17-8
1 suppliers
inquiry

6836-98-2
11 suppliers
$503.99/5MG