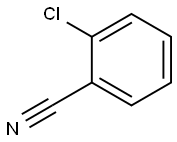
2-Chlorobenzonitrile synthesis
- Product Name:2-Chlorobenzonitrile
- CAS Number:873-32-5
- Molecular formula:C7H4ClN
- Molecular Weight:137.57
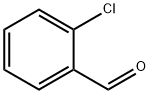
89-98-5
675 suppliers
$15.00/25g

873-32-5
568 suppliers
$9.00/1g
Yield:873-32-5 99%
Reaction Conditions:
with ammonium hydroxide;sodium persulfate;sodium iodide;iron(II) chloride in 1,2-dichloro-ethane at 20 - 50; for 16 h;
Steps:
General procedure for the synthesis of nitriles
General procedure: To a solution of aldehydes 1 (3 mmol) in 1,2-dichloroethane (10 mL) was added FeCl2(0.3 mmol), Na2S2O8 (4.5 mmol), NaI (0.15 mmol), and NH3.H2O (9 mL) at room temperature. After the mixture was stirred at 50 °C for 16 hours, it was poured into water (30 mL) and extracted with DCM (3 × 30 mL). The combined organic extracts were washed with brine and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the product 2.
References:
Chen, Han;Sun, Sijia;Xi, Haoying;Hu, Kaifang;Zhang, Ning;Qu, Jingping;Zhou, Yuhan [Tetrahedron Letters,2019,vol. 60,# 21,p. 1434 - 1436] Location in patent:supporting information
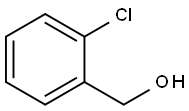
17849-38-6
293 suppliers
$6.00/10g

873-32-5
568 suppliers
$9.00/1g
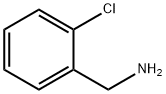
89-97-4
355 suppliers
$10.00/5g

873-32-5
568 suppliers
$9.00/1g
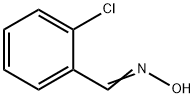
3717-28-0
109 suppliers
$15.00/5mg

873-32-5
568 suppliers
$9.00/1g
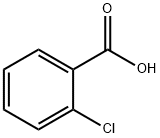
118-91-2
669 suppliers
$14.00/25g

873-32-5
568 suppliers
$9.00/1g