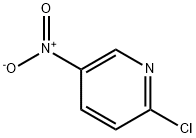
2-Chloro-5-nitropyridine synthesis
- Product Name:2-Chloro-5-nitropyridine
- CAS Number:4548-45-2
- Molecular formula:C5H3ClN2O2
- Molecular Weight:158.54
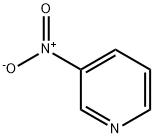
2530-26-9
100 suppliers
$29.69/1g

4548-45-2
557 suppliers
$6.00/5g
Yield:4548-45-2 98%
Reaction Conditions:
with hypochlorous anhydride;triethylamine;zinc(II) chloride;dinitrogen monoxide in dichloromethane at -15 - 20; for 4 h;Green chemistry;Reagent/catalyst;Temperature;Solvent;
Steps:
1-4 Example 2
In the specific implementation of the present invention,A preparation method for preparing 2-chloro-5-nitropyridine by using nitrous oxideAdding 12.4-g of 3-nitropyridine to the reaction vessel,Triethylamine 20.2g,Zinc chloride 16g,Mix 200mL of dichloromethane evenly,Ice salt bath to -15 ° C; at 0 ° C take 17 g of dichlorous oxide,Soluble in 100mL of dichloromethane,Then, the nitrous oxide solution is slowly added to the above reaction solution.The reaction solution is maintained at -10 ° C to 0 ° C,After the addition is completed within 2 hours,Warm up to room temperature and continue to stir 2Hours to complete reaction of the raw materials,Pour the reaction solution into 300 mL of ice water.After stirring for half an hour,Divide the organic layer,Collect organic layers,The organic layer was washed once with 100 mL of a 5% strength hydrochloric acid.Wash once in 100mL water,Dry over anhydrous magnesium sulfate,After filtering,Evaporate the solvent under reduced pressure.154g of yellow 2-chloro-5-nitropyridine solid,Yield 98%,The normalized purity of liquid chromatography is greater than 99%.
References:
CN108689920,2018,A Location in patent:Paragraph 0014; 0017-0024
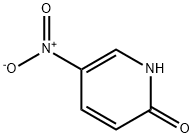
5418-51-9
455 suppliers
$12.72/1gm:

4548-45-2
557 suppliers
$6.00/5g
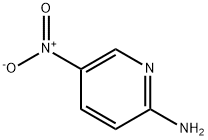
4214-76-0
525 suppliers
$6.00/10g

4548-45-2
557 suppliers
$6.00/5g
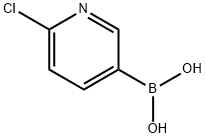
444120-91-6
278 suppliers
$6.00/1g

4548-45-2
557 suppliers
$6.00/5g
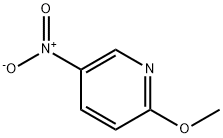
5446-92-4
314 suppliers
$10.00/5g

4548-45-2
557 suppliers
$6.00/5g