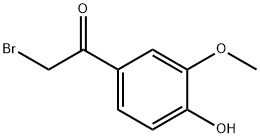
2-bromo-1-(4-hydroxy-3-methoxyphenyl)ethanone synthesis
- Product Name:2-bromo-1-(4-hydroxy-3-methoxyphenyl)ethanone
- CAS Number:69638-06-8
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07

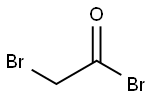
498-02-2
468 suppliers
$5.00/1g

69638-06-8
30 suppliers
inquiry
Yield:69638-06-8 80%
Reaction Conditions:
with copper(ll) bromide in ethanol at 70; for 1.5 h;Temperature;
Steps:
3.2.1; 3.2.2; 3.2.3 Synthesis of 2,4- (α-bromoacetyl) -guaiacol
(1) 7.0 g of cupric bromide was dissolved in 70 mL of absolute ethanol and transferred to a constant pressure funnel;(2) Add 5.0 g of 4-acetyl guaiacol into a three-necked flask containing 60 mL of absolute ethanol;(3)(1) was added dropwise to (2) at 70 ° C,About half an hour drops finished, observed the reaction color has been dark green,toAt the end of the reaction, a white cuprous bromide was observed and the progress of the reaction was monitored by TLC. The reaction time was about1.5 hours. After the reaction was completed, the reaction mixture was filtered through a sand funnel and the filtrate was evaporated under reduced pressure to remove ethanol.Extract with methylene chloride and water and dry the methylene chloride extract over anhydrous methylene sulfate. DichloromethaneThe extract was evaporated under reduced pressure to remove methylene chloride, resulting in dark red crystals. The yield is about 80%.
References:
CN106278832,2017,A Location in patent:Paragraph 0056-0059; 0071-0074; 0086-0089
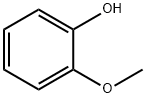
90-05-1
752 suppliers
$5.00/1g

69638-06-8
30 suppliers
inquiry