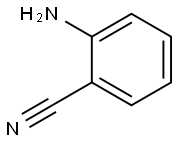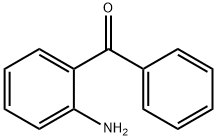
2-Aminobenzophenone synthesis
- Product Name:2-Aminobenzophenone
- CAS Number:2835-77-0
- Molecular formula:C13H11NO
- Molecular Weight:197.23
Yield:2835-77-0 88%
Reaction Conditions:
Stage #1:anthranilic acid nitrile;benzene with trifluorormethanesulfonic acid in dichloromethane at 60 - 80;Inert atmosphere;Houben-Housch synthesis;
Stage #2: with water in dichloromethaneInert atmosphere;Cooling with ice;Houben-Housch synthesis;
Stage #3: with sodium hydroxide in waterInert atmosphere;
Steps:
4.2. General preparation of the ketones
General procedure: The amino-nitrile (1 mmol) is dissolved in dry CH2Cl2 (5 mL) to which is added benzene (2 mL). With stirring, triflic acid (2 mL) is then slowly added and the reaction mixture is stirred overnight at 60-80 °C. The mixture is then quenched by pouring the solution over ice/water. The aqueous solution is then made basic by slow addition of 10 M NaOH and the solution is extracted twice with chloroform. The organic layer is washed with water, brine (2×20 mL) and dried over anhydrous sodium sulfate. In the event that imine/ketone mixtures are obtained, it may be necessary to stir the triflic acid/ice/water mixture for few hours in order to ensure complete hydrolysis. The products are purified via column chromatography (silica gel; hexanes/ether).
References:
Raja, Erum K.;Klumpp, Douglas A. [Tetrahedron,2011,vol. 67,# 25,p. 4494 - 4497] Location in patent:experimental part
7500-78-9
14 suppliers
inquiry

2835-77-0
447 suppliers
$8.00/5g
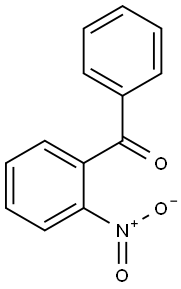
2243-79-0
36 suppliers
$44.00/250mg

2835-77-0
447 suppliers
$8.00/5g
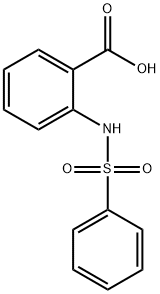
34837-67-7
28 suppliers
$74.00/1g

2835-77-0
447 suppliers
$8.00/5g