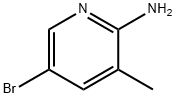
2-Amino-5-bromo-3-methylpyridine synthesis
- Product Name:2-Amino-5-bromo-3-methylpyridine
- CAS Number:3430-21-5
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
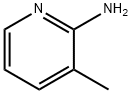
1603-40-3
491 suppliers
$6.00/25g

3430-21-5
400 suppliers
$5.00/1g
Yield:3430-21-5 100%
Reaction Conditions:
Stage #1:3-methylpyridin-2-ylamine with bromine in dichloromethane at 0 - 20; for 4.66667 h;
Stage #2: with sodium hydroxide in dichloromethane;water; pH=9
Steps:
Bromine (15.4 mL, 0.30 mol) was added dropwise to a stirred and cooled (0-2 [°C)] solution of 2-amino-3-picoline (1; 32.44 g, 0.30 mol) in dry [CH2CI2] (1000 mL) over a period of 1 h. The cooling bath was then removed, and the yellowish suspension was stirred at r. t. for 3 h 40 min. The mixture was basified to pH 9 with 2N [NAOH] (145 mL) followed by a mixture of saturated aqueous [NAHC03] (100 mL) -saturated aqueous Na2S203 (10 mL). The organic layer was separated and washed with brine, dried [MGS04] and concentrated to afford 14 (56.83 g, 102%) as brown solid, which was used in the next step without purification.
References:
EISAI CO., LTD WO2004/13139, 2004, A2 Location in patent:Page 25-26
7463-30-1
67 suppliers
$45.00/10mg

3430-21-5
400 suppliers
$5.00/1g