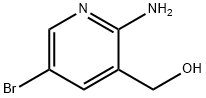
2-Amino-5-bromo-3-(hydroxymethyl)pyridine synthesis
- Product Name:2-Amino-5-bromo-3-(hydroxymethyl)pyridine
- CAS Number:335031-01-1
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04
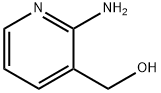
23612-57-9
209 suppliers
$8.00/250mg

335031-01-1
175 suppliers
$30.00/1/ G
Yield:335031-01-1 84%
Reaction Conditions:
with bromine in acetic acid at 20;Product distribution / selectivity;
Steps:
D1.2
Step 2: 2-Amino-5-bromo-3-(hydroxymethyl)pyridineBromine (8.4 mL, 189.4 mmol) was added dropwise over 1 hour to a solution of 2- amino-3-(hydroxymethyl)pyridine (19.6 g, 157.8 mmol; which may be prepared as described in Dl, Step 1) in acetic acid (350 mL) at room temperature. The reaction mixture was then stirred overnight. After concentration to dryness, the residue was partitioned between a saturated solution of potassium carbonate (300 mL) and ethyl acetate (200 ml_). The aqueous layer was separated and extracted with ethyl acetate (2 x 200 ml_). The combined organic phases were washed with a saturated solution of sodium chloride (200 ml_), dried over sodium sulfate, filtered and concentrated to dryness. After trituration of the residue in pentane, the title product was obtained as a yellow solid (27.0 g, 84%).*H NMR (DMSO-c/6, 400 MHz) : δ (ppm) : 7.90 (d, J = 2.8 Hz, 1H), 7.53 (d, J = 2 Hz, 1H), 5.93 (br s, NH2), 5.29 (br s, OH), 4.31 (s, 2H).
References:
WO2011/61214,2011,A1 Location in patent:Page/Page column 30-31
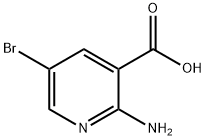
52833-94-0
212 suppliers
$13.00/1g

335031-01-1
175 suppliers
$30.00/1/ G
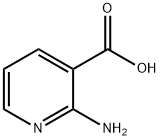
5345-47-1
378 suppliers
$9.00/1g

335031-01-1
175 suppliers
$30.00/1/ G