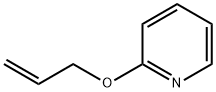
2-ALLYLOXYPYRIDINE synthesis
- Product Name:2-ALLYLOXYPYRIDINE
- CAS Number:5831-77-6
- Molecular formula:C8H9NO
- Molecular Weight:135.16
Yield:5831-77-6 95%
Reaction Conditions:
with 18-crown-6 ether;potassium hydroxide in toluene; for 2 h;Inert atmosphere;Reflux;
Steps:
2-Allyloxypyridine.
A 3-necked, 500 mL, round-bottomed flask was equipped with stir bar, reflux condenser fitted with a N2 inlet adapter, and 2 glass stoppers. Allyl alcohol (5.80 mL, 88.1 mmol), followed by 2-chloropyridine (5.00 mL, 52.7 mmol), and toluene (105 ml) were added sequentially to the flask. Potassium hydroxide (85%, 12.0g, 176 mmol) was ground in a mortar in pestle and then added to the reaction flask, followed by 18-crown-6 (0.278 g, 1.04 mmol). The reaction mixture was heated at reflux (oil bath at 115 °C) for 2h. After cooling to room temperature, the reaction mixture was diluted with diethyl ether (150 mL), washed with water (50 mL), followed by brine (50 mL). The organic layer was dried thoroughly over anhydrous sodium sulfate, and concentrated in vacuo to a pale yellow liquid. Column chromatography of the residue (29:1 hexanes/ethyl acetate) yielded the product as a pale yellow oil (6.73 g, 49.8 mmol, 95%)
References:
Strayer, Timothy A.;Culy, Caleb C.;Bunner, Matthew H.;Frank, Amie R.;Albiniak, Philip A. [Tetrahedron Letters,2015,vol. 56,# 48,p. 6807 - 6809] Location in patent:supporting information
20173-55-1
0 suppliers
inquiry

5831-77-6
8 suppliers
inquiry
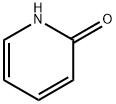
142-08-5
513 suppliers
$6.00/5g
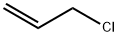
107-05-1
420 suppliers
$16.80/100ML
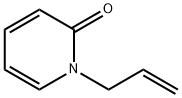
21997-30-8
18 suppliers
$60.00/100mg

5831-77-6
8 suppliers
inquiry