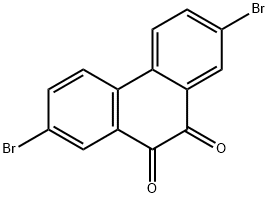
2,7-Dibromo-9,10-phenanthrenedione synthesis
- Product Name:2,7-Dibromo-9,10-phenanthrenedione
- CAS Number:84405-44-7
- Molecular formula:C14H6Br2O2
- Molecular Weight:366
84-11-7
253 suppliers
$5.00/1g

84405-44-7
140 suppliers
$16.00/1g
Yield:84405-44-7 90%
Reaction Conditions:
with sulfuric acid;hydrogen bromide;bromine in water at 80; for 24 h;
Steps:
1 Synthesis of 9,10:9,10-bis(trimethylene)-2,7-dibromo-9,10-dihydrophenanthrene
3 grams of phenanthrene-9,10-diketone (manufactured by Aldrich Co., 95%) was dissolved in 60 ml HBr and 20 ml H2SO4, and heated to 80° C., and a small amount of Br2 (manufactured by ACROS Co.) was added slowly, after which the mixture was allowed to react for 24 hours. After precipitation and filtration, dibromophenanthrene-9,10-diketone was obtained at a yield of more than 90%. 2 grams of NaOH and 200 ml methanol was mixed and heated to about 60° C. After NaOH was dissolved completely, 3 grams of dibromophenanthrene-9,10-diketone and 4 grams of diethyl 1,3-acetonedicarboxylate (manufactured by ACROS Co., 95%) were added and the temperature was maintained at 60° C. After 36 hours of reaction, 10% HCl aqueous solution was added to the reaction mixture for neutralization, and then the mixture precipitated and filtered. The precipitate collected was dissolved by acetic acid, 300 ml 10% HCl aqueous solution was added, and reaction took place for 18 hours at an elevated temperature. Then, acetic acid and water were removed, and the product neutralized with sodium hydrogen carbonate aqueous solution, precipitated, and filtered, giving Reactant 1 at a yield of 17%. The following reaction scheme illustrates the preparation of Reactant 1: 3 grams of Reactant 1 and 150 ml ethylene glycol were mixed, 0.5 grams of N2H4 (manufactured by Lancaster Co., 98%) was added, and the resulting mixture was stirred for 10 minutes, then 0.5 grams of KOH was added, and heated to about 180° C. for reaction. After 15 hours, the reaction mixture was cooled to room temperature and diluted with water, resulting in a white solid product (Compound 1), at a yield of 61%, herein referred as 9,10:9,10-bis(trimethylene)-2,7-dibromo-9,10-dihydrophenanthrene. This Compound 1 is the novel phenanthrene compound of the present invention, and is also a mediate for the synthesis of various phenanthrene compounds of the present invention. 1H NMR (CDCl3): δ (ppm) about 1.40 (m, 2H), about 1.70 (m,2H), 1.88-1.96(m, 4H), 2.10-2.16(m, 4H), 7.23-7.26(m, 2H), 7.23-7.26(m, 2H), 7.92(s, 2H). The following reaction scheme illustrates the preparation of Compound 1:
References:
Tuan, Chi-Shen;Tsai, Zong-Wei;Hsu, S-Sling;Cheng, Han-Bin;Cheng, Yu-Fen;Chang, Shinn-Jen US2005/176952, 2005, A1 Location in patent:Page/Page column 5
62325-30-8
101 suppliers
$158.00/200mg

84405-44-7
140 suppliers
$16.00/1g
61650-86-0
8 suppliers
inquiry

84405-44-7
140 suppliers
$16.00/1g
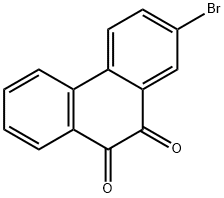
53622-33-6
24 suppliers
inquiry

84405-44-7
140 suppliers
$16.00/1g
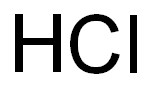
7647-01-0
0 suppliers
$10.00/10g
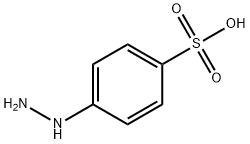
98-71-5
212 suppliers
$5.00/5g

84-11-7
253 suppliers
$5.00/1g

84405-44-7
140 suppliers
$16.00/1g