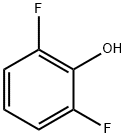
2,6-Difluorophenol synthesis
- Product Name:2,6-Difluorophenol
- CAS Number:28177-48-2
- Molecular formula:C6H4F2O
- Molecular Weight:130.09
5509-65-9
494 suppliers
$10.00/10g

28177-48-2
314 suppliers
$15.00/5 g
Yield:28177-48-2 88.9%
Reaction Conditions:
Stage #1:2,6-difluoroaniline with sulfuric acid;sodium nitrite in water at -5 - 0; for 2 h;Green chemistry;Large scale;
Stage #2: with sulfuric acid;copper(II) sulfate in waterReflux;Green chemistry;Large scale;Reagent/catalyst;Temperature;
Steps:
1.2; 2-8 (2) Preparation of aqueous phase of 2,6-difluorophenol:
25 kg of prepared 30% aqueous sulfuric acid solution was added to the reactor and3.0 kg (23 mol) of 2,6-difluoroaniline, heated under stirring,Completely dissolve 2,6-difluoroaniline and cool to -5 °C with vigorous stirring.Then slowly add 5.6 kg of prepared 30% sodium nitrite solution.Maintain the reaction temperature at -5 to 0 ° C, continue to react for 2 h after the addition, filter, discard the filter residue, decompose excess nitrous acid with aqueous urea solution, and adjust to the starch blue potassium iodide test paper.The 2,6-difluoroaniline diazonium salt aqueous solution was directly stored in the following reaction.Adding to a mixture of 22 kg of a 50% aqueous solution of sulfuric acid and 4.0 kg of copper sulfate in a separate kettle under reflux, and distilling while dropping, the resulting 2,6-difluorophenol is promptly transferred and distilled. The liquid was separated, dried, filtered, and evaporated under reduced pressure to give white product 2, 6-difluoro phenol with a purity of 98.6% and a yield of 88.9%.
References:
Ludong University;Liu Gang;Wang Haojie;Wang Jinming;Ren Yujin;Zhang Yuchun;Mu Xianfeng;Zhu Fei;Wang Shengjie;Han Qinglin;Xu Guanhua CN109456225, 2019, A Location in patent:Paragraph 0010; 0013; 0014; 0017-0023
437-82-1
214 suppliers
$18.00/1g

28177-48-2
314 suppliers
$15.00/5 g
121602-93-5
231 suppliers
$8.00/5g

28177-48-2
314 suppliers
$15.00/5 g
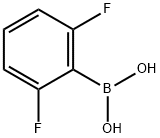
162101-25-9
334 suppliers
$6.00/1g

28177-48-2
314 suppliers
$15.00/5 g
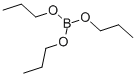
688-71-1
131 suppliers
$45.00/25mL
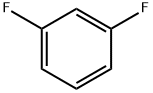
372-18-9
427 suppliers
$9.00/1g

28177-48-2
314 suppliers
$15.00/5 g