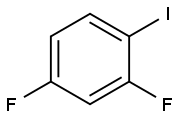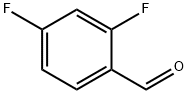
2,4-Difluorobenzaldehyde synthesis
- Product Name:2,4-Difluorobenzaldehyde
- CAS Number:1550-35-2
- Molecular formula:C7H4F2O
- Molecular Weight:142.1
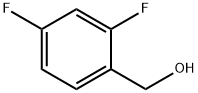
56456-47-4
200 suppliers
$5.00/1g

1550-35-2
380 suppliers
$6.00/5g
Yield:1550-35-2 89%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;copper diacetate in water;acetonitrile at 20; for 6 h;Green chemistry;
Steps:
General procedure: A mixture of alcohol (5.0 mmol), Cu(OAc)2 (9.1 mg, 0.05 mmol), and TEMPO (7.8 mg, 0.05 mmol) in CH3CN/H2O (5/10 mL) was stirred at room temperature for specified time. After completion of the reaction (monitored by TLC, eluents: petroleum ether/ethyl acetate = 4/1), dichloromethane (10 mL) was added to the resulting mixture. The dichloromethane phase was separated, and the aqueous phase was further extracted with dichloromethane (10 mL × 2). The combined organic layers were dried over anhydrous sodium sulfate and concentrated to give a residue, which was purified by column chromatography (eluents: petroleum ether/ethyl acetate = 10/1) to provide the desired product.
References:
Jiang, Jian-An;Du, Jia-Lei;Wang, Zhan-Guo;Zhang, Zhong-Nan;Xu, Xi;Zheng, Gan-Lin;Ji, Ya-Fei [Tetrahedron Letters,2014,vol. 55,# 10,p. 1677 - 1681] Location in patent:supporting information
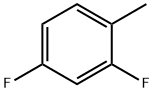
452-76-6
246 suppliers
$8.00/5g

1550-35-2
380 suppliers
$6.00/5g
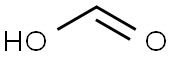
64-18-6
1088 suppliers
$22.12/250ML
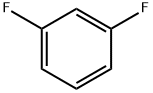
372-18-9
422 suppliers
$9.00/1g

1550-35-2
380 suppliers
$6.00/5g
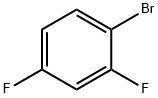
348-57-2
419 suppliers
$5.00/5g

1550-35-2
380 suppliers
$6.00/5g