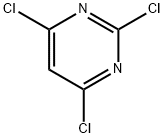
2,4,6-Trichloropyrimidine synthesis
- Product Name:2,4,6-Trichloropyrimidine
- CAS Number:3764-01-0
- Molecular formula:C4HCl3N2
- Molecular Weight:183.42
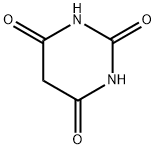
67-52-7
487 suppliers
$27.30/25g

3764-01-0
410 suppliers
$6.00/5g
Yield:3764-01-0 90%
Reaction Conditions:
with 1-methyl-pyrrolidin-2-one;anhydrous phosphorus trichloride;chlorine;trichlorophosphate
Steps:
2 Example 2
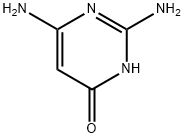
Example 2 Simultaneous addition 512 g (4 mol) of barbituric acid and 10 g of N-methylpyrrolidone were added to 3500 g (23,2 mol) of phosphorus oxychloride introduced in advance, and the mixture was then heated to 75+/-5° C. After a further stirring time of 7 hours, 1650 g (12.0 mol) of phosphorus trichloride and 840 g (11.8 mol) of chlorine were added and introduced simultaneously. After work-up by distillation, 661 g of 2,4,6trichloropyrimidine were obtained, corresponding to a yield of 90% of theory.
References:
Bayer Aktiengesellschaft US5898073, 1999, A
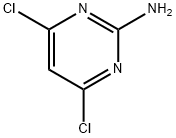
56-05-3
449 suppliers
$10.00/1g

3764-01-0
410 suppliers
$6.00/5g