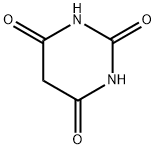
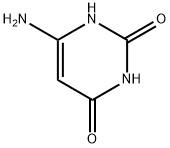
Barbituric acid synthesis
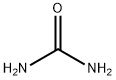
- Product Name:Barbituric acid
- CAS Number:67-52-7
- Molecular formula:C4H4N2O3
- Molecular Weight:128.09
Yield:67-52-7 70%
Reaction Conditions:
sodium methylate in ethylene glycol at 110; for 6 h;Conversion of starting material;
Steps:
15
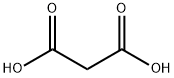
Barbituric acid Starting Materials Diethyl malonate 0.5 mol Barbituric acid Starting Materials Diethyl malonate 0.5 mol Urea 0.55 mol Sodium methoxide (catalyst) 0.5 mol Ethylene Glycol 223 g. Operating Conditions Pressure Atmospheric Temperature/time regime 110° C./6 h. Reaction Progress Monitored by TLC using Merck's silica gel plates, benzene- methanol (1:1) Work-up The reaction mixture was cooled to 60° C., diluted with 500 mL water at 50° C., made acidic (to a blue color with congo red indicator) by addition of 55.8 g. concentrated aqueous hydrochloric acid, refri- gerated overnight and filtered. The filter cake was washed with 50 mL cold (10° C.) water and dried at 90° C. during 4 h. 44.6 g, of white crystals, m.p. 245° C. (dec.) were obtained (literature 248° C. “with some decomposition”) Yield 70%
References:
US2005/27120,2005,A1 Location in patent:Page/Page column 8
504-17-6
418 suppliers
$10.00/5g

67-52-7
487 suppliers
$30.23/25g
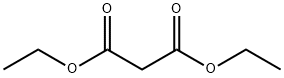
105-53-3
783 suppliers
$5.00/25g

67-52-7
487 suppliers
$30.23/25g
873-83-6
475 suppliers
$9.00/25g

67-52-7
487 suppliers
$30.23/25g