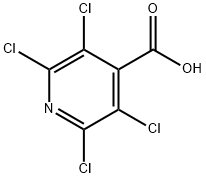
2,3,5,6-Tetrachloropyridine-4-carboxylic acid synthesis
- Product Name:2,3,5,6-Tetrachloropyridine-4-carboxylic acid
- CAS Number:19340-26-2
- Molecular formula:C6HCl4NO2
- Molecular Weight:260.89
2176-62-7
275 suppliers
$12.00/10g

124-38-9
130 suppliers
$175.00/23402

19340-26-2
31 suppliers
$144.00/1ea
Yield: 48%
Reaction Conditions:
Stage #1:2,3,4,5,6-pentachloropyridine with n-butyllithium in diethyl ether;hexane at -55; for 0.5 h;Inert atmosphere;
Stage #2:carbon dioxide in diethyl ether;hexane at -55 - 20;Inert atmosphere;
Steps:
1 Synthesis of Compound 7
To the pentachloropyridine (160 g, 0.63 mol, 1 equivalent) in ether solution (1.44 L) under N2 below -55° C., n-BuLi (2.5 M in hexane. 270 mL, 1.06 equivalent) was added dropwise. The mixture was stirred below -55° C. for 30 min. Dry CO2 was bubbled into the mixture and the temperature rose to room temperature. The mixture was acidified by adding conc. HCl (70 mL). The organic phase was separated and dried over Na2SO4, concentrated to dryness. The residue was suspended in refluxing DCM for 30 min, cooled down and filtered to get compound 7 as an off-white solid (80 g, 48% yield).
References:
X-Cutag Therapeutics, Inc.;LI, Hui Joyce;WEN, Shuhao;CHENG, Changfu;CHEN, Xiao;SHANG, Gao US2019/248790, 2019, A1 Location in patent:Paragraph 0075; 0076

84660-84-4
0 suppliers
inquiry

19340-26-2
31 suppliers
$144.00/1ea