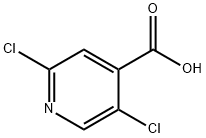
2,5-Dichloroisonicotinic acid synthesis
- Product Name:2,5-Dichloroisonicotinic acid
- CAS Number:88912-26-9
- Molecular formula:C6H3Cl2NO2
- Molecular Weight:192
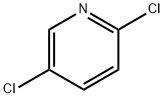
16110-09-1
467 suppliers
$5.00/1g

124-38-9
129 suppliers
$175.00/23402

88912-26-9
237 suppliers
$6.00/1g
Yield:88912-26-9 87%
Reaction Conditions:
Stage #1:2,5 dichloropyridine with n-butyllithium;N,N,N',N'',N'''-pentamethyldiethylenetriamine in tetrahydrofuran;hexane at -75; for 2 h;
Stage #2:carbon dioxide in tetrahydrofuran;hexane
Steps:
5A.A
2,5-Dichloro-4-pyridinecarboxylic acid was prepared according to known procedures (see Eur. J. Org. Chem. 2001, 1371-1376), as follows: At -75 °C, 2,5-dichloropyridine (3.7 g, 25 mmol) was added to a solution of butyllithium (1.6M in hexane) (25 mmol) and N,N,N',N",N"- pentamethyldiethylenetriamine (5.3 mL, 4.3 g, 25 mmol) in tetrahydrofuran (50 mL). After 2 h at - 75 °C, the mixture was poured onto an excess of freshly crushed dry ice. Water (50 mL) was10 added, the aqueous phase decanted and washed with diethyl ether (3 x 20 mL) and neutralized with 5N HCl to pH 1. The precipitate was filtered and washed with water to give 2.7g of white solid as a pure product. The filtrate was extracted with ethyl acetate and the combined organic layers were evaporated to dryness. The residue was recrystallized from ethanol to give another batch of pure product. (The filtrate was evaporated to small volume and the precipitate was filtered and washed15 with water to give another batch of product); m.p. 227-229 °C (from ethanol); 4.2 g (87%).[00294] 1H NMR (400MHz, DMSO-d6): a 14.43 (brs, 1H, exchangeable with D2O), 8.64 (s, 1 H), 7.87 (s, 1 H).[00295]2-(2,5-DicHoro-pyridke-4-carb∞yl)-3<(S)-1-hydroxymethyl-2-methyl-propylamino)- acrylic acid ethyl ester was synthesized using the same procedure described in example IA, (91 %20 yield).[00296] 1H NMR (DMSO-d6, 400MHz): δ 10.94 (dd, J=9.6 and 13.8Hz, 1H, NH, exchangeable with D2O), 8.48 (s, 1H), 8.28 (d, J=14.3Hz,1H, it becomes singlet after D2O exchange), 7.51 (s, 1H), 5.07 (t, J=5.1Hz,1H, OH, exchangeable with D2O), 3.92 (q, J=7.0Hz, 2H), 3.62 (m, 2H), 3.40 (m, 1H), 1.95 (m, 1H), 0.94 (d, J=6.6Hz, 3H), 0.91 (d, J=6.6Hz, 3H), 0.89 (t, J=7.0Hz, 3H). MS:25 375, 377 (M+l).
References:
ARDEA BIOSCIENCES INC. WO2009/89263, 2009, A2 Location in patent:Page/Page column 95
