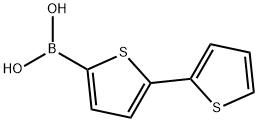
2,2'-BITHIOPHENE-5-BORONIC ACID synthesis
- Product Name:2,2'-BITHIOPHENE-5-BORONIC ACID
- CAS Number:132898-95-4
- Molecular formula:C8H7BO2S2
- Molecular Weight:210.08
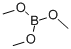
121-43-7
376 suppliers
$13.00/25mL
492-97-7
323 suppliers
$5.00/1g

132898-95-4
74 suppliers
$65.00/100 mg
Yield: 97%
Reaction Conditions:
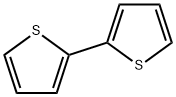
Stage #1:2,2'-Bithiophene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.25 h;
Stage #2:Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 3 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water
Steps:
72 Synthesis of (2,2′-bithiophen)-5-yl-boronic acid (109)
In a flame-dried, 3-neck, 250 mL rbf, 2,2′-bithiophene (5.0 g, 30.1 mmol) was dissolved in anhydrous THF (100 mL) and cooled to -78° C. (dry ice/acetone). A solution of n-BuLi in hexanes (2.5 M, 12.6 mL, 31.6 mmol) was added slowly over a period of 5 minutes. The reaction mixture was allowed to stir at -78° C. for 15 minutes, and then trimethyl borate (10.1 mL, 90 mmol) was added dropwise over a period of 5 minutes. The reaction was allowed to stir at -78° C. for 2 hours, then warm to RT and stir for a further 1 hour. The yellow reaction mixture was quenched by pouring it into a 10% HCl solution (250 mL). The mixture was extracted with ether (2×100 mL) and the combined organic portions were washed with water (500 mL), dried over MgSO4, filtered and solvent removed by rotary evaporation. The resulting yellow solid was washed with water, filtered and air dried to afford ( 109) (6.15 g, 97%). The material was used in the next step without further purification.
References:
SWITCH MATERIALS, INC.;Branda, Neil Robin;Finden, Jeremy Graham;Gauthier, Simon James;Hayek, Ali;Hope-Ross, Kyle Andrew;Senior, James Daniel;Spantulescu, Andreea;Sviridov, Serguei US2014/256936, 2014, A1 Location in patent:Paragraph 0446

492-97-7
323 suppliers
$5.00/1g

132898-95-4
74 suppliers
$65.00/100 mg
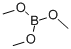
121-43-7
376 suppliers
$13.00/25mL

492-97-7
323 suppliers
$5.00/1g
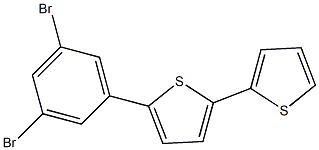
651329-37-2
0 suppliers
inquiry

132898-95-4
74 suppliers
$65.00/100 mg
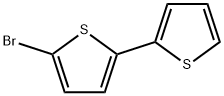
3480-11-3
75 suppliers
$11.00/100mg

132898-95-4
74 suppliers
$65.00/100 mg
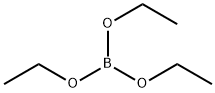
150-46-9
203 suppliers
$12.00/25mL

492-97-7
323 suppliers
$5.00/1g

132898-95-4
74 suppliers
$65.00/100 mg