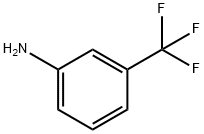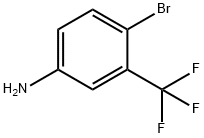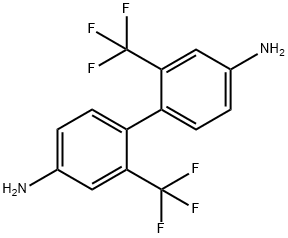
2,2'-Bis(trifluoromethyl)benzidine synthesis
- Product Name:2,2'-Bis(trifluoromethyl)benzidine
- CAS Number:341-58-2
- Molecular formula:C14H10F6N2
- Molecular Weight:320.23
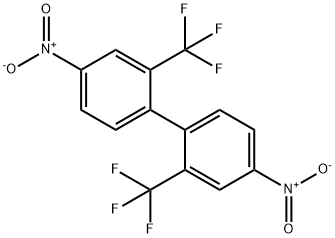
641-98-5
4 suppliers
inquiry

341-58-2
366 suppliers
$5.00/1g
Yield:341-58-2 98.8%
Reaction Conditions:
with Adams’s catalyst;hydrogen;palladium(0);2-hydroxyethylamine in tetrahydrofuran at 55; under 2250.23 Torr; for 2 h;Temperature;Pressure;
Steps:
1-7 Example 3: (The catalyst was applied twice)
100g of 2,2'-bis(trifluoromethyl)-4,4'dinitrobiphenyl was added to the reaction kettle, 500g of solvent tetrahydrofuran was added, 0.1g of new catalyst was added, and 0.1g of co-catalyst 2-aminoethanol was added. g, apply the wet weight 5g of the catalyst recovered in Example 2, and tighten the reaction kettle. Fill with nitrogen to test for leaks, and after confirming that there is no leak point, turn on stirring to carry out nitrogen replacement and hydrogen replacement. When the temperature was raised to 55°C, the hydrogen pressure was 0.3 MPa, and the reaction was continued for 1 h. The pressure of the reaction kettle basically did not change, and the temperature was kept for 1 h. After the reaction is completed, hot filtration is carried out, and the filter cake is washed with the hot solvent tetrahydrofuran, put back into the reaction kettle, and applied in the next batch. The filtrate was stirred and slowly cooled to 5°C, and white crystals were obtained through crystallization, filtration and washing. The obtained white crystals were dried under reduced pressure to obtain 2,2'-bis(trifluoromethyl)-4,4'diaminobiphenyl. Detection content 99.84%,The molar yield reached 98.8%,
References:
CN114957015,2022,A Location in patent:Paragraph 0039-0055
![Hydrazine, 1,2-bis[3-(trifluoromethyl)phenyl]-](/CAS/20180601/GIF/351-19-9.gif)
351-19-9
0 suppliers
inquiry

341-58-2
366 suppliers
$5.00/1g
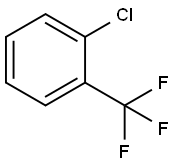
88-16-4
273 suppliers
$13.00/25g

341-58-2
366 suppliers
$5.00/1g
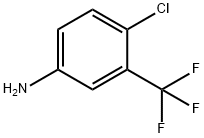
320-51-4
475 suppliers
$6.00/10g

341-58-2
366 suppliers
$5.00/1g