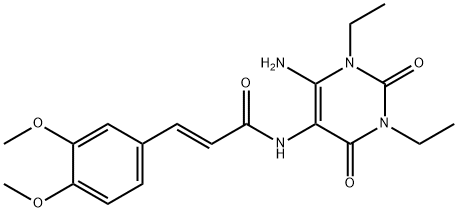
(E)-N-(6-aMino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyriMidin-5-yl)-3-(3,4-diMethoxyphenyl)acrylaMide synthesis
- Product Name:(E)-N-(6-aMino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyriMidin-5-yl)-3-(3,4-diMethoxyphenyl)acrylaMide
- CAS Number:187393-68-6
- Molecular formula:C19H24N4O5
- Molecular Weight:388.42
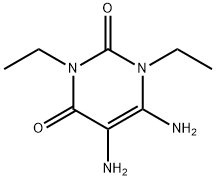
52998-22-8
85 suppliers
$199.00/250mg
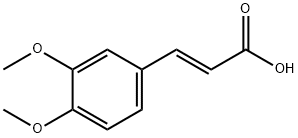
14737-89-4
137 suppliers
$30.00/25g

187393-68-6
26 suppliers
inquiry
Yield:187393-68-6 1016 g
Reaction Conditions:
Stage #1: 3,4-dimethoxy-trans-cinnamic acidwith oxalyl dichloride in dichloromethane;N,N-dimethyl-formamide at 25; for 1.5 h;
Stage #2: 5,6-diamino-1,3-diethyluracil in dichloromethane;N,N-dimethyl-formamide at 20; for 1 h;
Steps:
2 Example 2
Into a 10 L reaction flask, 605.2 g (2.91 mol) of starting material SM-I was added, 3.5 L of dichloromethane and 6.5 mL of DMF were added, and after stirring at 25C, 369.4 g (2.91 mol) of oxalyl chloride was added dropwise in 20 minutes. After stirring at 25 °C for 1.5 hours, the solution was clear. The reaction solution was slowly added dropwise to the aqueous solution of YN-II obtained in Step 1, and the temperature of the reaction solution was controlled at 0 to 5°C. After completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour and monitored by TLC (DCM:MeOH=50:1, UV 254 nm). The YN-II reaction was complete.Light yellow solidIt was filtered, recrystallized from 50% ethanol, and the resulting pale yellow solid was dried at 60° C. overnight to give Intermediate YN-III (1016 g) in 90% yield.
References:
CN107915735,2018,A Location in patent:Paragraph 0017; 0018
89073-60-9
107 suppliers
inquiry

187393-68-6
26 suppliers
inquiry
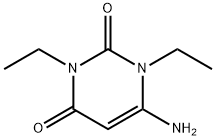
41740-15-2
82 suppliers
$135.00/500mg

187393-68-6
26 suppliers
inquiry
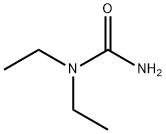
634-95-7
68 suppliers
$65.00/5G

187393-68-6
26 suppliers
inquiry

14737-89-4
137 suppliers
$30.00/25g

187393-68-6
26 suppliers
inquiry
