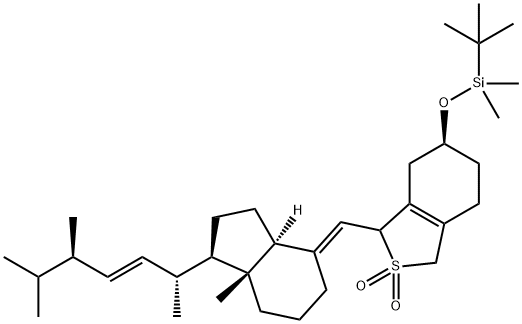
tert-Butyl-dimethyl-{3-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidenemethyl]-2,2-dioxo-2,3,4,5,6,7-hexahydro-1H-2l6-benzo[c]thiophen-5-yloxy}-silane synthesis
- Product Name:tert-Butyl-dimethyl-{3-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidenemethyl]-2,2-dioxo-2,3,4,5,6,7-hexahydro-1H-2l6-benzo[c]thiophen-5-yloxy}-silane
- CAS Number:170081-43-3
- Molecular formula:C34H58O3SSi
- Molecular Weight:574.97
Yield:170081-43-3 97%
Reaction Conditions:
Stage #1: Calciferolwith sulfur(IV) oxide in water monomer;toluene at 20; for 12 h;
Stage #2: tert-butyldimethylsilyl chloridewith 1H-imidazole in dichloromethane at 20; for 12 h;
Steps:
4.2. (6S)-1-{[(1R,3aS,4E,7aR)-1-[(1R,3E,5R)-5,6-dimethylhept-3-en-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]methyl}-6-[(tert-butyldimentylsilyl)oxy]-1,3,4,5,6,7-hexahydro-2l∧6-benzothiophene-2,2-dione (a1)
The synthesis of a1 is as previously described [42]. Briefly, to astirred solution of VD2 (5 g, 12.6 mmol) in toluene (100 mL) wasadded a sulfur dioxide solution portionwise (>6% in water, 150 mLin total). The mixture was vigorously stirred at RT for 12 h. Thereaction mixture was extracted with EtOAc. The organic layer waswashed with brine and saturated NaHCO3, then dried over Na2SO4.After concentrating under reduced pressure, the resultant residuewas redissolved in DCM (100 mL). Imidazole (3.95 g, 58.0 mmol)was added to the crude mixture in an ice-cold bath, followed byaddition of TBSCl (3.8 g, 25.2 mmol) and the mixture stirred at RTfor 12 h. The mixture was partitioned between DCM and brine,dried over Na2SO4, and concentrated under reduced pressure. Theresultant residue was purified by silica gel column chromatography(Eluent: 5%e20% EtOAc in Hex) to give a1 as a yellowish solid (7.0 g,97%). TLC (Hex:EtOAc 4:1): Rf 0.6, 0.5; 1H NMR (500 MHz,Chloroform-d) d 5.28e5.14 (m, 2H, H-22,23), 4.79e4.63 (m, 1H, H-7), 4.60e4.53 (m, 1H, H-6), 4.04e3.95 (m, 1H, H-3), 3.74e3.60 (m,2H, H-19), 2.66e2.52 (m, 1H), 2.32e2.17 (m, 1H), 2.17e1.96 (m, 5H),1.89e1.81 (m, 2H), 1.79e1.61 (m, 4H), 1.59e1.41 (m, 3H), 1.40e1.25(m, 3H), 1.04e1.01 (m, 3H, H-18), 0.92 (s, 9H, H-TBS), 0.89e0.86 (m,6H, H-25,27), 0.85e0.82 (m, 5H), 0.66e0.58 (m, 3H, H-18),0.11e0.04 (m, 6H, H-TBS); 13C NMR (126 MHz, CDCl3) d 150.64,135.52, 132.04, 130.69, 126.40, 110.10, 67.63, 66.48, 58.10, 56.34,56.15, 46.09, 42.89, 40.24, 34.25, 33.07, 30.68, 29.86, 27.69, 25.80(3C), 24.23, 23.93, 22.05, 21.12, 19.97, 19.65, 18.06, 17.67,12.00, 3.55, 4.73; LRMS: m/z 598.0 [MNa].
References:
Wen, Jiachen;Hadden, M. Kyle [European Journal of Medicinal Chemistry,2022,vol. 228,art. no. 114005]
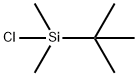
18162-48-6
692 suppliers
$9.00/5g
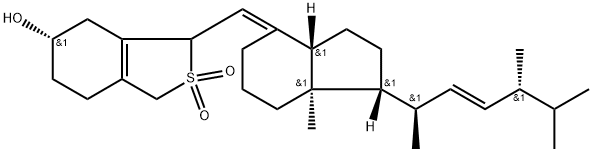
87680-65-7
18 suppliers
$460.00/50mg
![tert-Butyl-dimethyl-{3-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidenemethyl]-2,2-dioxo-2,3,4,5,6,7-hexahydro-1H-2l6-benzo[c]thiophen-5-yloxy}-silane](/CAS/GIF/170081-43-3.gif)
170081-43-3
8 suppliers
inquiry
![tert-Butyl-dimethyl-(4-methylene-3-{2-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidene]-ethylidene}-cyclohexyloxy)-silane](/CAS/GIF/104846-62-0.gif)
104846-62-0
15 suppliers
inquiry
![tert-Butyl-dimethyl-{3-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidenemethyl]-2,2-dioxo-2,3,4,5,6,7-hexahydro-1H-2l6-benzo[c]thiophen-5-yloxy}-silane](/CAS/GIF/170081-43-3.gif)
170081-43-3
8 suppliers
inquiry
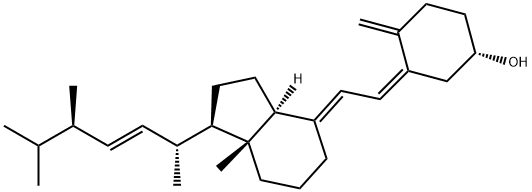
50-14-6
686 suppliers
$25.00/1g
![tert-Butyl-dimethyl-{3-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidenemethyl]-2,2-dioxo-2,3,4,5,6,7-hexahydro-1H-2l6-benzo[c]thiophen-5-yloxy}-silane](/CAS/GIF/170081-43-3.gif)
170081-43-3
8 suppliers
inquiry