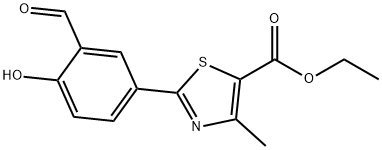
Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate synthesis
- Product Name:Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
- CAS Number:161798-01-2
- Molecular formula:C14H13NO4S
- Molecular Weight:291.32
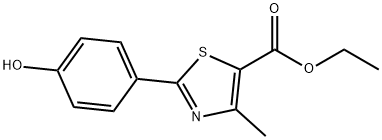
161797-99-5
372 suppliers
$10.00/1g
100-97-0
0 suppliers
$14.00/250G

161798-01-2
385 suppliers
$6.00/250mg
Yield:161798-01-2 91.2%
Reaction Conditions:
with methanesulfonic acid;boric acid in cyclohexane at 75 - 100; for 7 h;Duff Aldehyde Synthesis;Temperature;Reagent/catalyst;Solvent;
Steps:
1-19 Example 13: Preparation of ethyl 2- (3-carbaldehyde-4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylate
(1) Add 1 mole portion of the reactant 2- (4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylic acid ethyl ester (actual dosage 80g) to (in a 1L reaction bottle) preheated to 100 In a homogeneous mixture of 1.5 molar parts of polyphosphoric acid and 2 molar parts of methanesulfonic acid, and 0.05 molar parts of cyclohexane and 0.002 molar parts of boric acid, stir well;
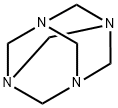
(2) To the above reaction mixture, 1.2 moles of Duff reaction reagent hexamethylenetetramine is added with stirring,Continue the reaction at a temperature of 75 ° C for 7 hours,After the reaction is completed, the reaction mixture is cooled to 38 ° C;
(3) Slowly add saturated ice brine (to about 80% of the total volume of the reaction flask) to the cooled reaction mixture,After the solid is precipitated, it is filtered, and the solid is washed with water until neutral and dried.The pale yellow solid is ethyl 2- (3-carbaldehyde-4-hydroxyphenyl) -4-methyl-5-thiazolecarboxylate,The yield was 91.2%.
References:
CN111039891,2020,A Location in patent:Paragraph 0109-0184

161797-99-5
372 suppliers
$10.00/1g
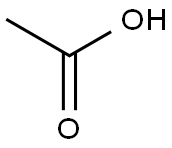
64-19-7
1584 suppliers
$10.00/25ML

161798-01-2
385 suppliers
$6.00/250mg
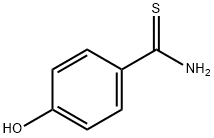
25984-63-8
500 suppliers
$8.00/10g

161798-01-2
385 suppliers
$6.00/250mg
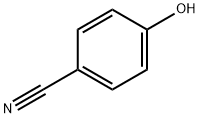
767-00-0
573 suppliers
$6.00/25g

161798-01-2
385 suppliers
$6.00/250mg
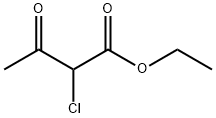
609-15-4
457 suppliers
$6.00/25g

161798-01-2
385 suppliers
$6.00/250mg