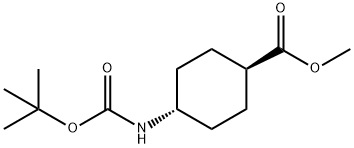
Methyl trans-4-(tert-butoxycarbonylamino)cyclohexanecarboxylate synthesis
- Product Name:Methyl trans-4-(tert-butoxycarbonylamino)cyclohexanecarboxylate
- CAS Number:146307-51-9
- Molecular formula:C13H23NO4
- Molecular Weight:257.33

24424-99-5
846 suppliers
$13.50/25G
62456-15-9
67 suppliers
$29.00/250mg

146307-51-9
109 suppliers
$6.00/100mg
Yield:146307-51-9 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 2.16667 h;Cooling with ice;
Steps:
3.1
Amine (132 g, 401 mmol) was suspended in dichloromethane (1000 ml) under ice-cooling. Triethylamine (123 ml, 882 mmol) and (Boc)2O (101 ml, 440 mmol) were sequentially added thereto and stirred for 10 minutes. After that, the mixture was stirred at room temperature for 2 hours and the solvent was removed. The residue was poured into aqueous citric acid (citric acid monohydrate 50 g in water 400 ml) to become pH4 and extracted with ethyl acetate. The organic layer was washed with water and dried over magnesium sulfate anhydrous. The solvent was removed under reduced pressure to quantitatively give the target compound.1H-NMR (DMSO-d6) δ ppm: 1.06-1.25 (m, 2H), 1.25-1.43 (m, 2H), 1.37 (s, 9H), 1.75-1.94 (m, 4H), 2.19 (tt, 1H, J=11.7, 3.9 Hz), 3.07-3.24 (m, 1H), 3.58 (s, 3H), 6.74 (d, 1H, J=6.6 Hz).
References:
US2010/273841,2010,A1 Location in patent:Page/Page column 21
15177-67-0
225 suppliers
$6.00/1g
75-65-0
760 suppliers
$10.00/10ml

146307-51-9
109 suppliers
$6.00/100mg
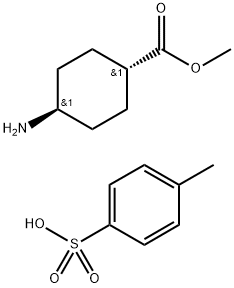
600133-26-4
0 suppliers
inquiry

24424-99-5
846 suppliers
$13.50/25G

146307-51-9
109 suppliers
$6.00/100mg
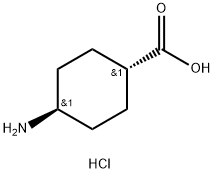
27960-59-4
152 suppliers
$17.00/1g

146307-51-9
109 suppliers
$6.00/100mg
![1,5-Anhydro-2,3,4-trideoxy-2-[[(1,1-diMethylethoxy)carbonyl]aMino]-D-erythrohexitol](/CAS/GIF/603130-12-7.gif)
603130-12-7
63 suppliers
$30.00/100mg

146307-51-9
109 suppliers
$6.00/100mg